Trump’s early tough talk on drug pricing is now a pro-industry, anti-regulation GOP dreamscape
By Bryn Gay
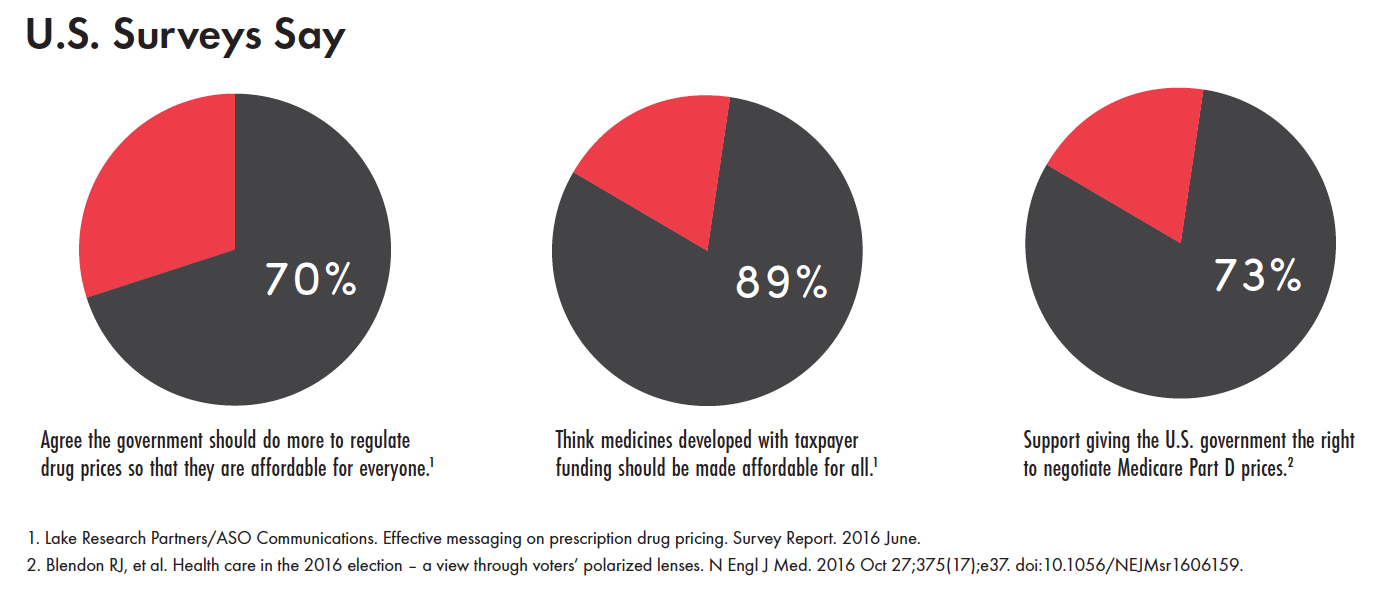
Unaffordable drug prices were a hot-button topic on the 2016 U.S. presidential campaign trail and remain a significant source of frustration among Democrats and Republicans in Congress. This common ground, backed by public opinion (see sidebar), can be leveraged to steer drug-pricing legislation and regulations toward truly bold initiatives that prioritize affordable treatment access.
Within his first two weeks in office, Trump met with pharmaceutical executives and regurgitated classic capitalist remedies for high drug pricing. Rather than consulting with patient groups or health policy specialists, the administration has primarily focused on meeting with the pharmaceutical industry. Trump’s initial stance of empowering the U.S. government to step up its ability to negotiate drug prices, notably for Medicare, have since shifted toward fast-tracking drug approval and rolling back regulations.
 Trump’s proposals fall short of and diverge from popular demands because they lack the ambition of approaches used in other countries to rein in drug cost expenditures: consolidation of purchasing power and other initiatives that would contribute to lower prices and earlier generic competition, such as shortened patent life; increased transparency in research and development (R&D) costs and pricing; and open-source research that discloses scientific findings, deprioritizes patent protection, and avoids monopolistic pricing. Executive action on drug pricing, however, appears unlikely.
Trump’s proposals fall short of and diverge from popular demands because they lack the ambition of approaches used in other countries to rein in drug cost expenditures: consolidation of purchasing power and other initiatives that would contribute to lower prices and earlier generic competition, such as shortened patent life; increased transparency in research and development (R&D) costs and pricing; and open-source research that discloses scientific findings, deprioritizes patent protection, and avoids monopolistic pricing. Executive action on drug pricing, however, appears unlikely.
Value-based drug-pricing regulations, such as those established by Germany’s AMNOG law, are another model that could potentially guide U.S. drug pricing reform and reward scientific innovation without bankrupting payers or patients. AMNOG determines pricing decisions on the basis of independent assessment of a new drug’s additional clinical benefits over existing drugs, relies on full transparency in all negotiation steps, and negotiates directly with pharmaceutical companies and key stakeholders, rather than involving government intermediaries. However, value-based pricing raises additional significant questions. Is a market-based economic system the best way to value drugs and human lives? Is the methodology of value-based tendering in itself transparent and does it reflect patients’ concerns? Does it address true innovation, or could ‘value’ be potentially rigged by the pharmaceutical industry?
U.S. Congressional attempts to permit pharmacies and patients with prescriptions to import low-cost medicines from Canada and other countries have popular support. However, federal initiatives, such as the bipartisan Safe and Affordable Drugs from Canada Act, face pushback from PhRMA, the pharmaceutical industry’s formidable lobbying group.
State houses are also taking up legislation to control drug costs. If these initiatives pass and prove to be effective, they could provide a template for other states and ultimately create a network of state-based resistance to high drug pricing. Maryland’s bill would require companies to disclose their R&D, manufacturing, and other costs, and would grant the state attorney general the authority to prosecute companies that price gouge ‘essential generics’ (for example, in the cases of Daraprim and Epipen). New York’s Governor has introduced legislation supporting value-based determinations (including R&D costing) for high-priced specialty drugs, along with provisions targeting the anti-competitive practices of pharmacy benefit managers—insurance intermediaries that collect rebates from manufacturers while passing exorbitant out-of-pocket costs on to consumers.
There may be opportunities at the international level that expand generic access to hepatitis C virus (HCV) treatments, including to the pangenotypic sofosbuvir/velpatasvir. Recent patent oppositions in India and Argentina have shown the strength of solidarity and economic cooperation in ensuring generic production of these medicines for countries in the global South. During the Trump years, we can anticipate the rise of fast-growing countries such as Brazil, Russia, India, China, and South Africa (BRICS) to finance drug development as well as to scale up their generic manufacturing and pooled procurement for medicines to get better pricing deals (for a review of other drug pricing strategies, see the Spring 2016 issue of TAGline).
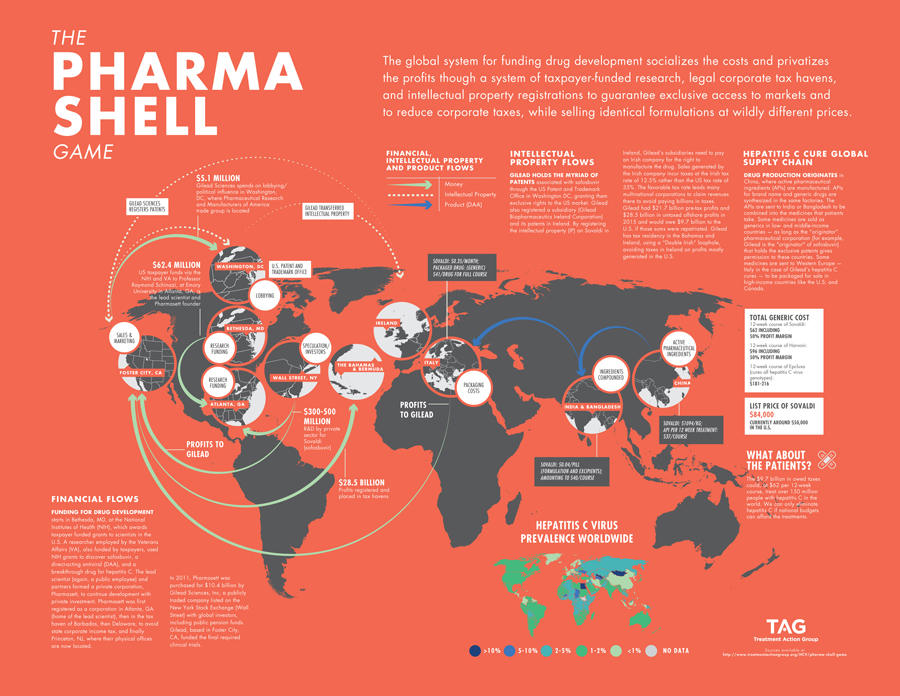
Another notable strategy, put forth by Peter Bach of Memorial Sloan Kettering Cancer Center and Mark Trusheim of Massachusetts Institute of Technology, recommends that the U.S. government buy Gilead for $156 billion to reduce spending on HCV drugs by one-third. Although certainly provocative, it does not consider actual R&D and production costs and is unsustainable for creating a more equitable drug development system. The complexity of funding drug development socializes the costs through taxpayers funding the bulk of biomedical research and privatizes profits through legal loopholes such as corporate tax havens and monopolistic pricing through strict intellectual property ownership. The pharmaceutical industry misleads the public by arguing that it needs to set high benchmark prices to recoup R&D expenses. In the case of Gilead, R&D expenses were already recouped in the first quarter of 2014 when sofosbuvir hit the market. Data from the research firm GlobalData revealed that the industry spent the majority of revenue on sales, marketing, advertising, and lobbying. Meanwhile, Dr. Andrew Hill and colleagues at the University of Liverpool showed that the actual cost to produce a drug, with a 50% profit, is often significantly less. The supply chain of sofosbuvir illustrates the pharmaceutical industry charade that prioritizes shareholders and profits over patients’ lives (see The Pharma Shell Game).
U.S. commitments to eliminate HCV will require affordable treatment for all patients, particularly those in the baby boomer and younger injection-drug-user cohorts. Evidence-based health policy that integrates drug-pricing solutions will be vital for overcoming treatment barriers. TAG will use this lens to engage in drug-pricing debates under the new White House administration. Our domestic and international networks, technical expertise, commitment to treatment literacy and inclusion of patients in policy-making, and critical voice will contribute to advocacy that informs state and federal legislation and regulation, debunks pharmaceutical industry myths, and rallies the base for securing affordable, high-quality medicines.