July 2017
By Annette Gaudino
INTRODUCTION
The continued development of direct-acting antivirals (DAAs) against hepatitis C virus (HCV) has brought both multigenotypic and pangenotypic regimens to market, with more on the horizon. These simpler-to- prescribe regimens potentially eliminate the need for genotype testing, have shown improved efficacy in previously difficult-to-treat patients, and hold the promise of massive scale up of treatment in primary care settings with nonspecialist providers, such as general internists and non-physicians, including nurse practitioners and physician assistants, as well as community pharmacists prescribing these therapies. Progress towards reliable, streamlined diagnostics that provide rapid confirmatory ribonucleic acid (RNA) testing has also continued, with manufacturers pursuing the goal of one-step point-of-care testing suitable for resource-limited settings. To effectively address the rising incidence of HCV among those who actively inject drugs, punitive approaches to drug use must be abandoned in favor of a public health approach, with people who use drugs at the center of the response.
Unless and until we can rapidly identify and treat chronically infected individuals, concrete progress towards the World Health Organization (WHO) targets on the elimination of HCV as a public health threat by 2030 will remain an elusive goal.1 Despite possessing highly effective short-course curative treatments that are the envy of those combatting HIV and TB, without unprecedented investment in implementation of strategic public health actions against HCV, we stand to miss a historic opportunity to wipe this deadly infectious disease from the face of the earth.
Global commitments are needed to end the HCV epidemic:
- National action plans with secure, multi-year funding for HCV treatment for everyone without restrictions, including treating reinfections;
- Sustainable global funding for generics, including multigenotypic and pangenotypic DAAs, and diagnostics in low- and middle-income countries;
- R&D for more options in point-of-care RNA assays to fill critical gaps in screening programs and put more patients on treatment;
- R&D for comprehensive diagnostic technologies that ensure rapid test results in a single visit, inform treatment regime choice, and confirm curative rates in patients;
- Research to develop a new class of DAAs to cure in four weeks;
- Continued funding for research towards a HCV vaccine that shows efficacy in people at risk for HCV infection because they inject drugs; the ability to elicit immune response in people living with HIV who are not at high risk for HCV infection; and safety in combination with HIV vaccine administration in healthy volunteers;
- R&D for dosage and effective treatment regimens for infants and children (aged 3-12 years) and weighing less than 35 kilograms (77 pounds);
- Post-treatment studies on the efficacy and long-term health effects for sofosbuvir and sofosbuvir/ ledipasvir in adolescents (aged 12-17 years);
- Expanded risk based screening beyond the birth cohort (1945-1965 in the U.S.; different ranges outside the U.S.)
- Decriminalization of drug use and centering the needs of those most at risk for infection.
Beyond blockbuster prices: adding tools to the toolkit
The arrival of highly effective, interferon-free, single daily dose DAAs in 2014 led to remarkably increased public awareness of HCV, but hasn’t led to a comprehensive response to the epidemic. The eye-popping price of Gilead’s essential compound sofosbuvir (Sovaldi) generated countless headlines and outrage as the latest example of corporate greed in the pharmaceutical industry. However, focus on the high price of HCV cures has dominated the public response to the epidemic, only slowly and haltingly generating movement on the public health challenge posed by HCV infection. Although calls to address the high price of pharmaceutical drugs have frequently used HCV cures as the exemplars of everything wrong with the status quo, the movement for drug-pricing reform has, overall, rarely engaged directly in the struggle for HCV treatment access. A broad coalition bringing together activists for patent law and drug development reform, drug user health and harm reduction, and those living with HCV could be a powerful force to demand action.
As advocates fight to be heard, recent approvals of multigenotypic and pangenotypic treatments continue to add tools to our anti-HCV toolkit. New and soon-to-be available options from multiple manufacturers not only benefit patients, especially those with advanced disease, co-morbidities, and difficult-to-treat genotype 3, but also offer payers needed flexibility when choosing regimens for their formularies. Drugs in the development pipeline, most notably AbbVie’s pangenotypic combo glecaprevir/pibrentasvir (Maviret), will go head to head with Gilead’s sofosbuvir/velpatasvir (Epclusa).
In high-income countries, Merck’s Zepatier (grazoprevir/elbasvir) and AbbVie’s Viekira Pak have been used as alternative, more-affordable regimes in patients with genotype 1 and 4. Viekira Pak is offered as a multi-pill twice daily regimen, and is not approved for patients with genotype 4 and cirrhosis.
Zepatier requires pre-treatment NS5A-resistance testing in patients with genotype 1a. A new once daily formulation of AbbVie’s four-drug combination, Viekira XR, was approved in July 2016, and appears to be a more attractive option for patients and providers. Gilead’s drugs are not currently available through state AIDS Drug Assistance Programs (ADAP) for HIV/HCV co-infected patients in the U.S., potentially allowing AbbVie to leverage their position in ADAP formularies for their new pangenotypic combo G/P when it hits the U.S. market.
Pending drugs in the pipeline: the dawn of the pangenotypic era
AbbVie, Gilead, Merck, and Janssen have presented data at international congresses on efficacy across the six major genotypes; in difficult-to-treat populations, including patients with genotype 3 and cirrhosis; and patients with advanced kidney disease. Gilead also recently received approval for previously untreated adolescents, and presented data on ongoing clinical trials in young children. It would not be hyperbole to state that science has solved chronic HCV infection for all but individuals with decompensated cirrhosis—yet another powerful argument for early treatment. It must be noted that, as historically has been the case, all clinical trial data is based on majority male patient populations, with few people of color, particularly African Americans, taking part in clinical trials.
Table 1. Multigenotypic and Pangenotypic DAAs in the pipeline
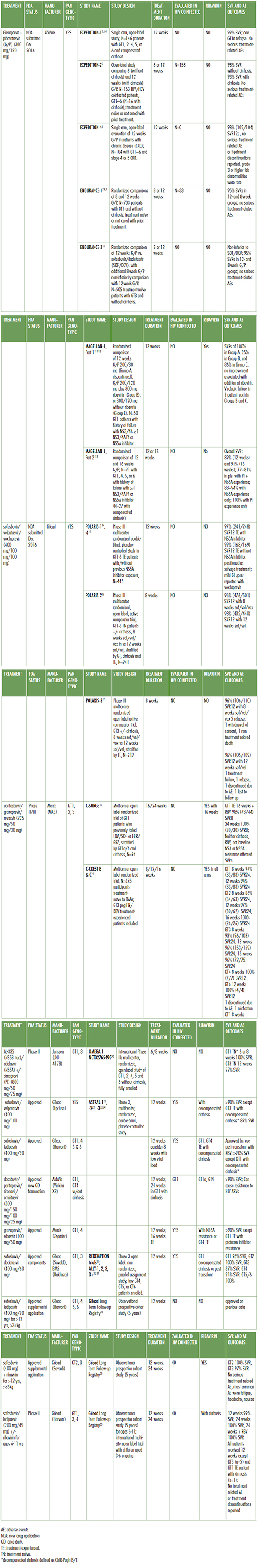
Treatment duration: how short can we go?
Shortening treatment duration has been of interest to patients and providers since the development of interferon-based treatments. Cost is usually understood as the unstated reason for seeking to go shorter. For example, cost savings may have motivated recent real-life studies of eight-week courses of ledipasvir/sofosbuvir at the Veterans Administration.31 However, drug prices are not based on the costs of pill production,32 so the clinical benefits of shorter treatment courses must be clear and significant. Policy makers and providers perceive that adherence to daily oral treatment over 12 weeks will be too challenging for patients who lack stable housing, or are actively using illicit substances. In practical terms, reducing treatment length from 12 to eight or six weeks still requires a return trip to the pharmacy, as DAAs are typically dispensed in 30-day supplies. Thus, the ability to reliably achieve SVR12 with 4 weeks/28 days would be a significant breakthrough for some vulnerable patients.
The current class of drugs have similar chemical kinetics, suggesting that a new class of compounds would be needed to achieve SVR12 with only four weeks of DAA treatment.33 Viral load at four weeks of treatment is strongly correlated with SVR12 post-treatment.34 According to some experts, one bottle—and one trip to the pharmacy—is likely the physiological limit to eliminate HCV.35 Results for new compounds from Gilead and Merck demonstrate that reliably successful eight-week treatments are here, particularly for patients without cirrhosis. However, 6-week treatment courses have not yet demonstrated high SVR12 rates. Researchers should continue to explore shorter treatment courses with the goal to achieve SVR12 greater than 90% with four weeks of treatment.
Glecaprevir/Pibrentasvir
A new drug application for the fixed dose, once daily combination glecaprevir/pibrentasvir (G/P; Maviret) was submitted by AbbVie in December of 201636 with FDA approval anticipated in Quarter 3 2017. Registration trials in genotypes 1-6 demonstrated uniformly high 12-week sustained virologic response (SVR12) rates of 95% with eight weeks of treatment in treatment-naïve patients without cirrhosis.37 Difficult-to-treat patients with genotype 3, with and without cirrhosis, and patients with chronic kidney disease had SVR12 rates of 93-100%.38 Among patients with previous DAA failure due to baseline resistance associated substitutions (RASs) SVR12 was 94% with G/P.39
AbbVie has lagged in the number of patients treated behind Gilead and Merck, as well as generic formulations based on Gilead and Bristol-Meyers Squibb (daclatasvir) developed compounds in high- income countries.40 Pricing for G/P will ultimately determine treatment uptake for this promising new treatment.
Sofosbuvir/Velpatasvir/Voxilaprevir
While AbbVie was aiming at Gilead’s dominant treatments, Gilead was targeting salvage treatment for genotype (GT) 1–6 patients who had previously failed a DAA- or interferon-based regimen. The addition of a new NS3/4A protease inhibitor to sofosbuvir/velpatasvir resulted in SVR12 in 98% these patients with eight or 12 weeks of treatment (POLARIS trials).41 However, this new triple therapy, to be branded as Vosevi, has not been adequately tested in patients with decompensated cirrhosis. Mild gastro-intestinal upset, including nausea and diarrhea, were reported, but were not severe enough to discontinue treatment.
April 2017 saw the FDA approval for the use of Gilead’s Sovaldi and Harvoni in adolescents aged 12-17 years old, weighing more than 35 kilograms (77 pounds) without cirrhosis or with compensated cirrhosis.42 Sovaldi (sofosbuvir) in combination with weight-based ribavirin is indicated for adolescents with genotypes 2 and 3, also without cirrhosis or with compensated cirrhosis. Harvoni (sofosbuvir/ ledipasvir) is indicated for adolescents with genotypes 1, 4, 5 and 6, providing effectively pangenotypic treatment for this population with Gilead’s products. Clinical trials for children aged 3-12 years and weighing less than 35 kilograms are ongoing.43
Uprifosbuvir/Grazoprevir/Ruzasvir
Phase II data on a novel triple combination consisting of NS5B polymerase inhibitor uprifosbuvir (formerly known as MK-3682), approved protease inhibitor grazoprevir (component in Zepatier) and novel NS5A inhibitor ruzasvir (formerly MK-8408) have been presented by Merck.44 Also known as MK3, this once daily fixed-dose combination was studied against genotypes 1, 2 and 3 in treatment durations ranging from eight weeks to 24 weeks. GT1 patients achieved SVR12 at a rate of 95% (84/88, GT1a and GT1b) with 8 weeks and 98% (45/46) with 12 weeks of treatment, respectively. GT2 had limited response to eight weeks of treatment, with 86% (54/63) achieving SVR12. GT2 patients receiving 12 weeks of MK3 had 97% (60/62) and 100% (26/26) SVR12. Finally, GT3 patients responded with 95% (98/103) SVR12 with eight weeks, 97% (155/159) with 12 weeks and 96% (72/75) with 16 weeks. In summary, treatment duration of at least 8 weeks was sufficient to achieve high SVR12 rates with the exception of patients with genotype 2, who required 12 weeks.45 Significantly, neither the addition of ribavirin nor the presence of compensated cirrhosis impacted treatment outcomes.
AL-335/Odalasvir/Simeprevir
Development of a novel NS5B nucleoside analogue (AL-335) in combination with odalasvir (NS5A inhibitor), with and without simeprevir (protease inhibitor Olysio), continues as the result of a partnership between Achillion and Janssen. The triple combination is known as JNJ-4178, and preliminary Phase II results in treatment-naive and treatment-experienced patients without cirrhosis have been presented.46 Treatment-naive patients with genotype 1 and without cirrhosis who were treated with the triple combo for six or eight weeks resulted in 100% SVR24 (20/20 in each arm). Of patients with genotype 3 who relapsed during eight weeks of treatment, 77% achieved SVR12 when extended to 12 weeks. However, eight weeks of treatment was insufficient for GT3 patients, with only 77% (10/13) achieving SVR12 even when extended to 12 weeks on the triple combo.47 A Phase IIb study of efficacy in non-cirrhotic patients with genotypes 2, 4, 5, and 6 is ongoing.48
Injectables
Data on a proof-of-concept injectable micro-RNA (miRNA) based treatment from Merck was expected at the 67th Meeting of the American Association for the Study of Liver Diseases (AASLD) in 2016; however, the poster was withdrawn prior to the conference.49 The market viability of injectable treatments based on difficult-to-produce miRNA technology is questionable given the efficacy of current oral treatments, and the future development of this treatment route is unclear.
Generic DAAs
Real-world data on generic DAAs, most extensively sofosbuvir and daclatasvir in fixed-dose combination, have consistently demonstrated SVR12 rates comparable to those of drugs manufactured by originator companies (REDEMPTION trials).50 Patients accessing generics manufactured in Bangladesh, China, and India achieved an average SVR12 rate across all genotypes. As with branded sofosbuvir/daclatasvir, GT3 continued to be difficult-to-treat, achieving an SVR12 rate of only 94%. National health ministries should implement generic-based treatment for everyone wherever voluntary licenses are registered. Unfortunately, registration with national regulatory bodies continues to be a major barrier to treatment uptake in low- and middle-income countries, with expanded registration being a top priority among global treatment activists. Real-time data on registration is available at mapCrowd.org, a collaboration between Medécins du Monde and Treatment Action Group.
Real World Data in People Who Use Drugs
Transmission of HCV among people who inject drugs continues to be the main driver of the global epidemic. Stigmatization, discrimination and myths that active drug users cannot adhere to daily treatment regimens have resulted in treatment restrictions and other policies that further marginalize those we need to engage the most. However, post-marketing studies of DAA treatment in active drug users and those in opioid substitution therapy (OST) demonstrate that HCV cure rates comparable to those in clinical trials can be achieved among people who inject drugs.
The SIMPLIFY trial, a Phase IV open-label multicenter international trial of sofosbuvir/velpatasvir in people with injection drug use in the prior six months and compensated liver disease, resulted in 94% of participants achieving SVR12 (96/99; four participants were lost to follow up).51 Participants were recruited from March through October 2016 with no relapse or reinfection observed to date.
The C-EDGE CO-STAR trial, a Phase III randomized double blind parallel group trial of grazoprevir/ elbasvir in patients in OST for minimum of three months, consisted of two arms: 12 weeks of treatment versus placebo for 12 weeks followed by 12 weeks of treatment (starting at week 16).52 Both arms achieved high SVR12 rates: 96% (189/198) and 97% (85/88), respectively. Follow up continued to SVR24, with 96% (175/186) and 97% (82/85) of patients maintaining cure. The six reinfections which occurred are equivalent to 3.4 per 100 person years.
These data support treatment for everyone without restriction.
Finding people with chronic HCV infection: diagnostics for elimination
Globally, less than 5% of individuals chronically infected with viral hepatitis have been diagnosed, and estimates of the global burden of chronic HCV are 71–80 million individuals (POLARIS Observatory data).53 Modeling studies indicate that 5–10% of the global infected population must be treated each year from 2018–2030 to achieve the targeted 90% reduction in viral hepatitis incidence and 65% reduction in associated mortality. To screen and diagnose the hundreds of millions of individuals at risk of infection, diagnostic technologies and algorithms will need to be rethought, streamlined, and implemented across a range of settings outside of tertiary hospital or even primary care community clinic sites. To meet this tremendous need, technologies will need to be affordable, provide results in a single visit, sufficiently inform regimen choice, and confirm cure.
As concisely described by John Dillon, MD, Professor of Hepatology and Gastroenterology, University of Dundee, the minimal inputs for confirmed cure of HCV are blood for an RNA confirmatory test, DAAs, and blood to confirm SVR12. Ideally, blood could be collected as a dried blood spot. Building clinic infrastructure and deploying new technologies appropriate to acquire the blood inputs are critical priorities for the next three to five years. It is particularly important to deploy low-cost solutions in resource-limited settings across high-, middle-, and low-income countries, specifically where people who inject drugs receive harm reduction, opioid substitution, and other services; in jails and prisons; to migrants regardless of legal status; and where pregnant women receive care. In high-income countries, including the U.S., emergency rooms have been shown the potential of capturing new infections, particularly among young people outside of the baby boomer birth cohort (1945–1965 in the U.S.),54 but lack both the payer mechanisms and clinic flow to inform and link infected individuals to care in a timely manner. Rapid point-of-care RNA assays could fill critical gaps in screening programs and provide opportunities to effectively bring more into treatment.
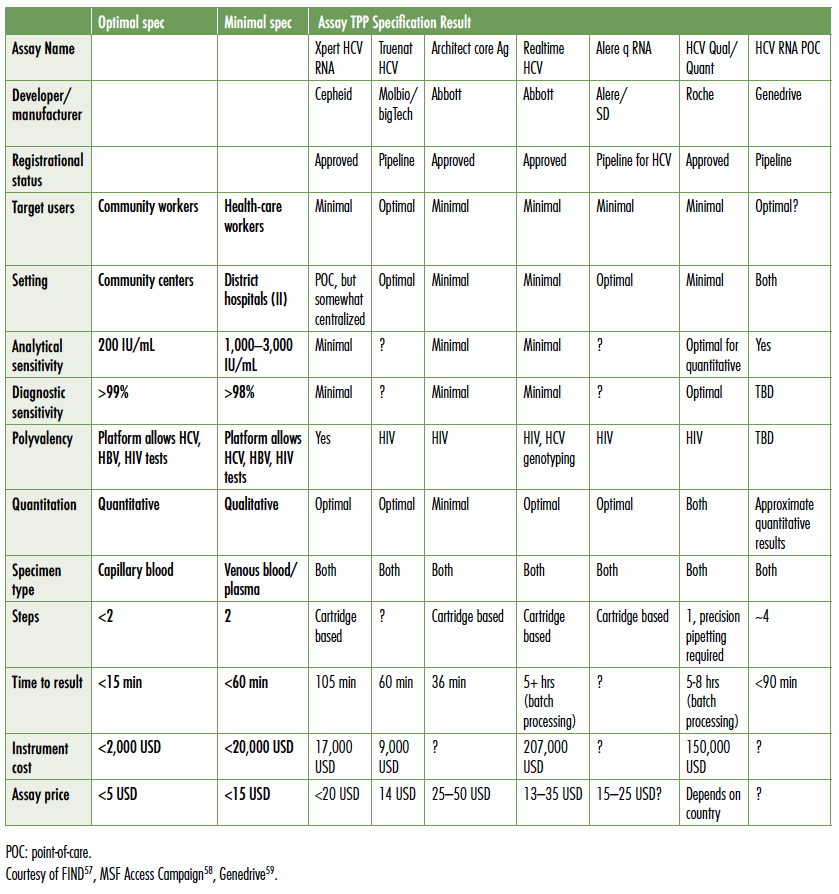
The only point-of-care rapid diagnostic HCV antibody test available in the field and recognized as reliable by regulatory bodies is the Oraquick test from Orasure. Although not yet WHO prequalified, the assay is CE marked (Conformité Européene; accepted as quality assured in the European Union) and provides results with both capillary blood and oral swabs. However, with pricing ranging from USD$8 to over USD$10, price remains a barrier to wide-scale deployment in limited resource settings. The work of Andrew Hill, University Liverpool, has shown that generic DAAs can be produced for less than USD$200 per treatment course, including 50% mark up.55 In countries with voluntary licensing, generic prices continue to fall, approaching Hill’s model. Ironically in those circumstances, pre- and post-treatment diagnostics can cost USD$500–600,56 with little or no support for patients. As a result, out-of-pocket diagnostic costs are often a greater barrier to treatment access than the price of DAAs.
The Foundation for Innovative New Diagnostics (FIND), the leading non-profit organization advocating for appropriate diagnostics in low- and middle-income countries, has developed target product profiles (TPPs) for HCV diagnostics. The following table compares point-of-care tests currently in the field and in the pipeline to FIND’s TPP (see Table 2).
Table 2. Target Product Profiles for HCV Diagnostic
HCV TREATMENT RECOMMENDATIONS
Next steps: getting where we want to go
Elimination of viral hepatitis C as a public health concern is feasible. Although we currently lack reliable, affordable diagnostics and nonspecialist provider capacity, those can be developed with time and commitment. Curative oral therapies continue to improve, and, as disease progression and sobriety restrictions fall, scaled up treatment and competition will contribute to driving prices down for branded and generic drugs.
Decades of research into the virus has yielded a promising vaccine candidate (see Box: A vaccine for HCV?) and real-world data on treatment adherence and shortened treatment duration suggests that it is possible to further cut costs and improve the efficiency of public health strategies. Implementation science on how to intervene successfully to prevent reinfection and support the most vulnerable patients—active injection drug users, the homeless, and other marginalized communities—will be critical in this phase of the fight.
Concrete, concerted action is needed to move forward:
- National governments must develop HCV action plans in consultation with affected populations, especially people who use drugs, people co-infected with HIV, and women;
- National governments must use every available legal tool, including TRIPS flexibilities, compulsory licenses, and patent opposition, to secure affordable DAAs;
- Generic producers and diagnostics manufactures must partner to develop bulk procurement proposals in low- and middle-income countries;
- Public and private payers must make multi-year commitments to fund HCV diagnosis and treatment;
- Diagnostic technologies and algorithms will need to be rethought, streamlined, and implemented across a range of settings outside of tertiary hospital or even primary care community clinic sites;
- More options for point-of-care RNA assays are needed to fill critical gaps in screening programs and facilitate putting more patients on treatment.
- Diagnostic technologies will need to be affordable for low- and middle-income countries and provide results in a single visit, sufficiently inform regimen choice, and confirm curative rates in patients;
- Sustainable funding for vaccine research to show efficacy in people at risk for HCV infection because they inject drugs; the ability to elicit immune response in individuals living with HIV who are not at high risk for HCV infection; and safety in combination with HIV vaccine administration in healthy volunteers;
- Researchers must pursue four-week treatment courses that match current SVR12 rates of 90% or greater across genotypes, levels of disease severity, and comorbidities and infections, including HIV/HCV co-infection.
Science has brought us to the brink of winning the biochemical battle with HCV. How far and how fast we move towards elimination will depend on evidence-based solutions to battles outside of the liver— the incoherence between intellectual property and the human right to health, the lack of global funding streams for viral hepatitis, the deadly war against drug users, and the need to galvanize global demand for treatment for all.
With thanks to Gregory Dore (Kirby Institute, University of New South Wales Sydney), David Bernstein (North Shore University Hospital and LIJ Medical Center), and Bryn Gay (Treatment Action Group).
A vaccine for HCV?
The world’s first recombinant vaccine was the hepatitis B vaccine, based on hepatitis B surface antigen, and a half dozen commercial vaccines exist for hepatitis A. However, a prophylactic HCV has eluded researchers. That may be about to change.
Although HIV is a more extreme example, HCV can also be considered a master virus, supremely adapted to stay one step ahead of the human immune system. Rapidly mutating and ten times more variable than HIV in its genotypic subtypes, HCV elicits a weak immune response,60 resulting in poor viral control and chronic infection of hepatocytes (liver cells) in most. Approximately 25% of individuals exposed to HCV spontaneously clear the virus. People who inject illicit drugs and other high-risk groups can be repeatedly exposed to HCV. Some individuals in in high-risk groups infected are repeatedly able to clear the virus again and again without treatment and exhibit a broadening of their adaptive antibody-mediated immune response with repeated exposure to HCV.
Evidence from people who control HCV infection and primate studies suggests a potential role for broadly neutralizing antibodies (bNAbs) in effective vaccine design for HCV. bNAbs for both HIV and HCV have been identified, and how to induce bNAbs is being studied for vaccine development. However, the precise interaction of antibody and T-cell-mediated responses in protecting against infection are unknown at this time. bNAbs targeting HCV envelope proteins have been tested in healthy people.61
Further back in the pipeline, the only vaccine ever tested in high-risk individuals is an HCV prophylactic vaccine (Ad Ch3 NS/MVA NS) originated by Okairos and that is now being developed in collaboration with GlaxoSmithKline. Results are pending from Phase II trials in three groups: for efficacy in people at risk for HCV infection because they inject drugs (NCT01296451); for ability to elicit immune response in individuals living with HIV (NCT02568332) who are not at high risk for HCV infection; and for safety in combination with HIV vaccine administration in healthy volunteers (NCT02362217). These results will determine whether this candidate vaccine is effective on its own or needs to be combined or enhanced with vaccines that generate bNAbs against envelope proteins.
The high cost of curative treatments will continue to limit treatment access in the near term, and cost-driven concerns about reinfection, particularly among people who inject drugs and men who have sex with men, present considerable challenges to advocates for universal access and treatment as prevention. Primary prevention through an affordable, effective vaccine could be our most powerful tool for defeating the virus. Data generated in this upcoming year will tell us if we’re one step closer to adding a preventative vaccine to our HCV toolkit.
With thanks to Andrea Cox (Johns Hopkins University).
REFERENCES
- World Health Organization. Global health sector strategy on viral hepatitis, 2016–2021. Geneva: World Health Organization; 2016 June. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf
- Forns X, Lee S, Valdes J, et al. EXPEDITION-I: Efficacy and safety of glecaprevir/pibrentasvir for treatment of chronic hepatitis C virus genotype 1, 2, 4, 5 or 6 infection in adults with compensated cirrhosis. Paper presented at: 52nd International Liver Congress; 2017 April 19-22; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_11.htm.
- Hassanein T, Wyles D, Wang S, et al. SURVEYOR-II, Part 4: glecaprevir/pibrentasvir [ABT493+ABT530] demonstrates high SVR rates in patients with HCV genotype 2, 4, 5, or 6 infection without cirrhosis following an 8-week treatment duration. Paper presented at: AASLD; 2016 Nov 11-15; Boston. http://www.natap.org/2016/AASLD/AASLD_52.htm.
- Puoti M, Foster GR, Wang S, et al. High SVR rates with eight and twelve weeks of pangenotypic glecaprevir/pibrentasvir: integrated efficacy analysis of genotype 1-6 patients without cirrhosis. Paper presented at: 52nd International Liver Congress; 2017 April 19-23; Amsterdam, Netherlands. http://natap.org/2017/EASL/EASL_38.htm.
- Rockstroh J, Lacombe K, Viani RM, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients coinfected with hepatitis C virus and human immunodeficiency Virus-1: the EXPEDITION-2 study (Abstract LBP-522). Paper presented at: 52nd International Liver Congress; 2017 April 19-23; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_148.htm.
- Gane E, Lawitz E, Pugatch D, et al. EXPEDITION-4: Efficacy and safety of glecaprevir/pibrentasvir (ABT-493/ABT-530) in patients with renal impairment and chronic hepatitis C virus genotype 1 – 6 infection. Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_31.htm.
- Stefan Z, Jordan F, Stanley W, et al. ENDURANCE-1: a phase 3 evaluation of the efficacy and safety of 8- versus 12-week treatment with glecaprevir/pibrentasvir (formerly ABT-493/ABT-530) in HCV genotype 1 infected patients with or without HIV-1 co-infection and without cirrhosis. Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_32.htm.
- Kowdley KV, Colombo M, Zadeikis N, et al. ENDURANCE-2: Safety and efficacy of glecaprevir/pibrentasvir in hepatitis C virus genotype 2-infected patients without cirrhosis: a randomized, double-blind, placebo-controlled study. Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_14.htm.
- Tarik A, Christophe H, Neddie Z, et al. ENDURANCE-4: Efficacy and safety of glecaprevir/pibrentasvir (formerly ABT-493/ABT-530) treatment in patients with chronic HCV genotype 4, 5, or 6 infection. Paper presented at: 52nd International Liver Congress; 2017 April 21; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_13.htm.
- Foster GR, Gane E, Asatryan, et al. ENDURANCE-3: Safety and efficacy of glecaprevir/pibrentasvir compared to sofosbuvir plus daclatasvir in treatment-naïve HCV genotype 3-infected patients without cirrhosis. Paper presented at: AASLD; 2016 Nov 11-15; Boston. http://www.natap.org/2017/EASL/EASL_13.htm.
- Poordad F, Pol S, Asatryan A, et al. MAGELLAN-1, Part 2: glecaprevir/pibrentasvir for 12 or 16 weeks in patients with chronic HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure. Paper presented at: 52nd International Liver Congress; 2017 April 19-22; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_27.htm.
- Poordad F, Felizarta F, Asatryan A, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatol. 2017 Jan 27. doi: 10.1002/hep.29081. [Epub ahead of print].
- Poordad F, Pol S, Asatryan A, et al. MAGELLAN-1, Part 2: glecaprevir/pibrentasvir for 12 or 16 weeks in patients with chronic HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure. Paper presented at: 52nd International Liver Congress; 2017 April 19-22; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_27.htm.
- Bourlière M, Gordon SC, Ramji A, et al. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks as a salvage regimen in NS5A inhibitor-experienced patients with genotype 1-6 infection: the phase 3 POLARIS-1 study. Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_29.htm.
- Sarrazin C, Cooper C, Manns MP, et al. No impact of RASs on the high efficacy of SOF/VEL/VOX for 12 weeks in DAA-experienced patients: an integrated resistance analysis of the POLARIS-1 and POLARIS-4 studies (Abstract THU-248). Paper presented at: 52nd International Liver Congress; 2017 April 19-23; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_57.htm.
- Jacobson IM, Asselah T, Nahass R, et al. A randomized phase 3 trial of sofosbuvir/velpatasvir/voxilaprevir for 8 weeks compared to sofosbuvir/velpatasvir for 12 weeks in DAA-naïve genotype 1-6 HCV infected patients: the POLARIS-2 study. Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_33.htm.
- Foster GR, Thompson A, Ruane PJ, et al. A randomized, phase 3 trial of sofosbuvir/velpatasvir/voxilaprevir for 8 weeks and sofosbuvir/velpatasvir for 12 weeks for patients with genotype 3 HCV infection and cirrhosis: the POLARIS-3 study. Paper presented at: AASLD; 2016 Nov 11-15; Boston. http://www.natap.org/2016/AASLD/AASLD_34.htm.
- Wyles D, Wedemeyer H, Reddy KR, et al. Safety and efficacy of the fixed-dose combination regimen of MK-3682/ grazoprevir/MK-8408 (ruzasvir) in cirrhotic or non-cirrhotic patients with chronic HCV GT1 infection who previously failed a direct-acting antiviral regimen (C-SURGE). Paper presented at: AASLD; 2016 Nov 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_28.htm.
- Lawitz E, Yoshida EM, Buti M, et al. Efficacy and safety of the fixed-dose combination regimen of grazoprevir/ruzasvir/ uprifosbuvir (MK-3682) with or without ribavirin in non-cirrhotic or cirrhotic participants with chronic HCV GT1, 2, 3, 4, or 6 infection (Parts A & B of C-CREST-1 & 2). Paper presented at: 52nd International Liver Congress; 2017 April 19-21; Amsterdam, Netherlands. http://natap.org/2017/EASL/EASL_05.htm.
- Gane E, Stedman C, McClure M, et al. Short Duration Treatment with AL-335 and Odalasvir, with or without simeprevir, in treatment naïve patients with hepatitis C infection with or without cirrhosis. Paper presented at: 52nd International Liver Congress, 2017 April 19-21; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_26.htm.
- Feld JJ, Agarwal K, Hezode C, et al. A phase 3 double-blind placebo-controlled evaluation of sofosbuvir/velpatasvir fixed-dose combination for 12 weeks in genotype 1, 2, 4, 5, 6 HCV-infected patients: results of the ASTRAL-1 study. Paper presented at: AASLD; 2015 Nov 13-15; San Francisco, CA. http://www.natap.org/2015/AASLD/AASLD_19.htm.
- Sulkowski M, Bräu N, Lawitz E, et al. A randomized controlled trial of sofosbuvir/velpatasvir fixed-dose combination for 12 weeks compared to sofosbuvir with ribavirin for 12 weeks in genotype 2 HCV-infected patients: the phase 3 ASTRAL-2 study. Paper presented at: AASLD; 2015 Nov 13-15; San Francisco, CA. http://www.natap.org/2015/AASLD/AASLD_62.htm.
- Mangia A, Roberts SK, Pianko R, et al. Ribavirin for 24 weeks in genotype 3 HCV-infected patients: the randomized controlled phase 3 ASTRAL-3 study. Paper presented at: AASLD; 2015 Nov 13-15; San Francisco, CA. http://www.natap.org/2015/AASLD/AASLD_63.htm.
- Charlton MR, O’Leary JG, Bzowej NH, et al. Sofosbuvir/velpatasvir fixed-dose combination for the treatment of HCV in patients with decompensated liver disease: the phase 3 ASTRAL-4 study/ASTRAL 1, 2 and 3. Paper presented at: AASLD; 2015 Nov 13-15; San Francisco, CA. http://www.natap.org/2015/AASLD/AASLD_64.htm.
- Freeman J, Khwairakpam G, Dragunova J, et al. Sustained virological response for 94% of people treated with low cost, legally imported generic direct acting antivirals for hepatitis C: analysis of 1087 patients in 4 treatment programmes (Abstract PS-097). Paper presented at: 52nd International Liver Congress; 2017 April 19-23; Amsterdam, Netherlands.
- Leroy V, Angus P, Bronowicki JP, et al. All-Oral Treatment With Daclatasvir Plus Sofosbuvir Plus Ribavirin for 12 or 16 Weeks in HCV Genotype 3-Infected Patients With Advanced Fibrosis or Cirrhosis: The ALLY-3+ Phase 3 Study. Paper presented at: 66th Liver Meeting, 2015 Nov 13-15; San Francisco. http://www.natap.org/2015/AASLD/AASLD_53.htm
- Wyles DL, Ruane P, Sulkowski M, et al. Daclatasvir in Combination With Sofosbuvir for HIV/HCV Coinfection: ALLY-2 Study. Paper presented at: CROI, 2015 Feb 23-26; Seattle. http://www.natap.org/2015/CROI/croi_47.htm
- Murray KF. Ledipasvir/sofosbuvir ± ribavirin for 12 or 24 weeks is safe and effective in children 6-11 years old with chronic hepatitis C infection (PS101) Paper presented at: 52nd International Liver Congress; 2017 April 19-23; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_22.htm
- Ibid.
- Ibid.
- Ioannou GN, Beste LA, Chang M, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir and paritaprevir/ritonavir/ ombitasvir and dasabuvir-based antiviral regimens in 17,847 patients in the Veterans Affairs National Healthcare System (Abstract 21). Paper presented at: AASLD; 2016 November 11-15; Boston, MA.
- Hill A, Pozniak A, Gotham D, Barber M, Fortunak J. Rapidly falling costs for new hepatitis C direct-acting antivirals (DAAs): Potential for universal access. Poster at: 21st International AIDS Conference; Durban, South Africa; 2016 July 18–22. http://www.natap.org/2016/IA/IAC_121.htm.
- Dahari H, Canini L, Graw F, et al. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir J Hepatol. 2016 June; 64(6): 1232–39.
- Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post- treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatol. 2015 Jan;61(1):41–5. doi: 10.1002/hep.27366
- Dahari H, Canini L, Graw F, et al. HCV kinetic and modeling analyses indicate similar time to cure.
- AbbVie. (Press Release). AbbVie submits new drug application to U.S. FDA for its investigational regimen of glecaprevir/ pibrentasvir (G/P) for the treatment of all major genotypes of chronic hepatitis C. 2016 December 19. Available from: https://news.AbbVie.com/news/AbbVie-submits-new-drug-application-to-us-fda-for-its-investigational-regimen-glecaprevirpibrentasvir-gp-for-treatment-all-major-genotypes-chronic-hepatitis-c.htm.
- Zeuzem S, Feld J, Wang S, et al. ENDURANCE-1: A phase 3 evaluation of the efficacy and safety of 8- versus 12-week treatment with glecaprevir/pibrentasvir (formerly ABT-493/ABT-530) in HCV genotype 1 infected patients with or without HIV-1 co-infection and without cirrhosis. Paper presented at: AASLD; 2016 November 11-15; Boston, MA. http://www.natap.org/2016/AASLD/AASLD_32.htm.
- Foster GR, Gane E, Asatryan A, et al. ENDURANCE-3: Safety and efficacy of glecaprevir/pibrentasvir compared to sofosbuvir plus daclatasvir in treatment-naïve hcv genotype 3-infected patients without cirrhosis. Paper presented at: 52nd International Liver Congress, 2017 April 19-21; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_13.htm.
- Reau N, Kwo PY, Rhee S, et al. MAGELLAN-2: Safety and efficacy of glecaprevir/pibrentasvir in liver or renal transplant adults with chronic hepatitis C genotype 1-6 infection. Paper presented at: 52nd; 2017 April 19-22; Amsterdam, Netherlands. http://www.natap.org/2017/EASL/EASL_32.htm.
- Silverman E. “Gilead hepatitis C pill was biggest 2015 drug cost for Medicare, Medicaid.” Stat [Internet]. 2016 November 14. Available from: https://www.statnews.com/pharmalot/2016/11/14/medicare-medicaid-gilead-hepatitis/
- Bourlière M, Gordon SC, Ramji A, et al. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks as a salvage regimen.
- Gilead. (Press Release). U.S. FDA approves new indications for Harvoni® and Sovaldi® in pediatric patients 12 years and older with chronic hepatitis C infection. 2017 April 7. Available from: http://www.gilead.com/news/press-releases/2017/4/us-fda-approves-new-indications-for-harvoni-and-sovaldi-in-pediatric-patients-12-years-and-older-with-chronic-hepatitis-c-infection.
- ClinicalTrials.gov [Internet]. Identifier NCT02249182, Safety and efficacy of ledipasvir/sofosbuvir fixed dose combination +/-ribavirin in adolescents and children with chronic HCV-infection; 2017 April. https://clinicaltrials.gov/ct2/show/NCT02249182.
- Lawitz E, Yoshida EM, Buti M, et al. Efficacy and safety of the fixed-dose combination regimen of grazoprevir/ruzasvir/ uprifosbuvir (MK-3682) with or without ribavirin in non-cirrhotic or cirrhotic participants with chronic HCV GT1, 2, 3, 4, or 6 infection (Parts A & B of C-CREST-1 & 2). Paper presented at: 52nd International Liver Congress, 2017 April 19-21; Amsterdam, Netherlands. http://natap.org/2017/EASL/EASL_05.htm.
- Merck (Press Release). Merck announces findings for investigational triple-combination chronic hepatitis C therapy showing high rates of sustained virologic response in people with genotypes 1, 2 or 3 infection. 2016 November 13. http://www.businesswire.com/news/home/20161113005036/en/Merck-Announces-Findings-Investigational-Triple-Combination-Chronic-Hepatitis.
- Gane E, Stedman C, McClure M, et al. Short duration treatment with AL-335 and odalasvir, with or without simeprevir, in treatment naïve patients with hepatitis C infection with or without cirrhosis. Paper presented at: 52nd International Liver Congress, 2017 April 19–21; Amsterdam, Netherlands. http://natap.org/2017/EASL/EASL_26.htm.
- Ibid.
- ClinicalTrials.gov [Internet]. Identifier NCT02765490, efficacy and safety of combinations of AL-335, odalasvir (ODV) and simeprevir (SMV) in the treatment of chronic hepatitis C infection; 2017 May, https://clinicaltrials.gov/ct2/show/NCT02765490.
- Govath H, Papatheodoridis GV, Fabri MJ, et al. Poster #111 submitted to: AASLD; 2016 November 11-15; Boston, MA. doi/10.1002/hep.28796.
- Freeman J, Khwairakpam G, Dragunova J, et al. Sustained virological response for 94% of people treated with low cost, legally imported generic direct acting antivirals for hepatitis C: analysis of 1087 patients in 4 treatment programmes (Abstract PS-097). Paper presented at: 52nd International Liver Congress; 2017 April 19–23; Amsterdam, Netherlands. http://fixhepc.com/blog/item/79-easl-2017-generics-results.html.
- Grebely J, Dalgard O, Conway B, et al. Efficacy and safety of sofosbuvir/velpatasvir in people with chronic hepatitis C virus infection and recent injecting drug use: the SIMPLIFY study. Paper presented at: 52nd International Liver Congress; 2017 April 19-22, Amsterdam, Netherlands. http://natap.org/2017/EASL/EASL_30.htm.
- Dore GJ, Altice F, Litwin AH, et al. Efficacy of elbasvir/grazoprevir fixed-dose combination for 12 weeks in HCV-infected persons who inject drugs on opioid agonist therapy. Paper presented at: 51st International Liver Congress; 2016 April 13-17, Barcelona, Spain. http://www.natap.org/2016/EASL/EASL_107.htm.
- World Health Organization. Global hepatitis report. Geneva: World Health Organization; 2017 April. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1.
- Galbraith J, Hsieh Y-H, Rothman R, et al. National Viral Hepatitis Roundtable Webinar: Hepatitis C screening in emergency departments. Presented online at National Viral Hepatitis Roundtable; 2016 September 26. http://nvhr.org/content/nvhr-webinar-hepatitis-c-screening-emergency-departments.
- Hill A, Pozniak A, Gotham D, Barber M, Fortunak J. Rapidly falling costs for new hepatitis C direct-acting antivirals (DAAs): Potential for universal access. Poster at: 21st International AIDS Conference.
- Medécins Sans Frontières (MSF). Putting HIV and HCV to the test: a product guide for point-of-care CD4 and laboratory- based and point-of-care virological HIV and HCV tests. 2015 July. https://www.msfaccess.org/sites/default/files/HIV_HCV_Report_Diagnostic_Guide_2015.pdf.
- The Foundation for Innovative New Diagnostics (FIND). High-priority target product profile for hepatitis C diagnosis in decentralized settings: Report of a consensus meeting. 2015 April. https://www.finddx.org/target-product-profiles/.
- Medécins Sans Frontières (MSF). Putting HIV and HCV to the test: a product guide for point-of-care CD4 and laboratory- based and point-of-care virological HIV and HCV tests. 2015 July. https://www.msfaccess.org/sites/default/files/HIV_HCV_Report_Diagnostic_Guide_2015.pdf.
- Genedrive. Genedrive HCV ID kit documentation. 2017 April. https://www.genedrive.com/assays/hcv-documentation.php.
- Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterol. 2010 Jan; 138: 315–24. doi: 10.1053/j.gastro.2009.09.017.
- Frey SE, Houghton M, Coates S, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010 Aug; 28(38): 6367–73. doi: 10.1016/j.vaccine.2010.06.084.
