Immunologic nonresponders face increased risk of illness, but lack therapeutic options
By Richard Jefferys
A subset of people on antiretroviral therapy (ART) experience limited or no recovery of CD4 T-cell counts despite achieving and maintaining undetectable viral loads. Various terms have been used to describe this phenomenon, with the most common being “immunological nonresponders” (INRs). Many studies have documented that INRs face an increased risk of illness and death compared with people with more robust CD4 T-cell gains. Yet there are no approved therapies to improve immune reconstitution, and clinical trials of potential candidates remain few and far between.
Approximately 5–10% of individuals initiating ART have been characterized as INRs, usually based on minimal change from baseline levels or failure to reach a threshold (e.g., 350 cells) after several years of viral suppression. The most prominent risk factors are a low CD4 T-cell count at ART initiation and older age.
A number of biological mechanisms that contribute to inadequate CD4 T-cell recovery in INRs have been identified. These include compromised production of CD4 T cells (and other lymphocytes) in the bone marrow and reduced output of T cells from the thymus. Both bone marrow and thymus function also decline naturally with age, likely explaining why older age is a risk factor.
CD4 T-cell survival is also shortened in INRs due to heightened activation of the immune system (driving cells into a short-lived activated state) and fibrotic damage to lymph node structures that normally provide sustenance to CD4 T cells via the cytokine IL-7. Potential contributors to heightened immune activation in INRs include coinfections such as CMV and hepatitis C, and microbial translocation (the leakage of gut bacteria into the systemic circulation due to reduced immune surveillance in the intestine).
Multiple cohort studies in the Americas, Europe, and Africa have assessed clinical outcomes among INRs followed over several years. The results have been very consistent: the overall rates of illness and death among individuals with sustained HIV suppression are relatively low, but the risk of both AIDS- and non-AIDS-related events is significantly increased compared to individuals with superior CD4 T-cell count recovery (see figure).
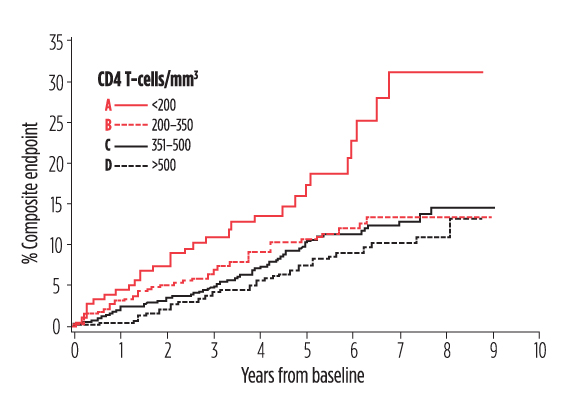
Poor CD4 T-cell recovery, despite suppression of viral load, was associated with an increased risk of death, AIDS, cancer, liver disease, and cardiovascular events (composite endpoint) in the Dutch ATHENA HIV cohort. Compared with Group A, Group D had a 46% reduced risk of the composite endpoint. Group B had a 66% reduced risk of the composite endpoint. Adapted from van Lelyveld et al. AIDS. 2012 Feb 20;26(4):465-474.
These findings jibe with those from analyses showing that individuals on ART who achieve CD4 T-cell counts over 500 show mortality relates comparable to uninfected individuals, while those who do not continue to face a shortfall in life expectancy.
So far only one large-scale trial (named SILCAAT) has tested whether an adjunctive therapy can reduce illness and death among INRs on ART. The study started in 1999 and tested IL-2, a cytokine that had been shown to stimulate CD4 T-cell proliferation, but it failed to show any clinical benefits. Subsequent analyses revealed that IL-2 preferentially increased numbers of a type of immune-suppressive regulatory CD4 T cell, emphasizing that not all CD4 T cells are created equal, and that the mechanism of action of an immune-based therapy can be crucial to whether it confers improvements in health.
An array of small studies have explored the immunologic effects of other possible therapeutic candidates; among those that have fallen by the wayside are the CCR5 inhibitor maraviroc and a putative thymus-enhancer named keratinocyte growth factor. Human growth hormone (HGH) looked promising in one trial, boosting thymus volume and CD4 T-cell production, but is widely viewed as having too many potentially serious side effects to be worthy of further consideration.
Among the candidates that appear to have most promise are Sangamo BioSciences’ SB-728-T gene therapy, which involves extracting CD4 T cells from individuals, modifying them in the laboratory to abrogate expression of the HIV coreceptor CCR5, then expanding and reinfusing them. Phase I studies in INRs reported sustained CD4 T-cell count increases, unprecedented improvements in CD4 to CD8 T-cell ratios, and anecdotal evidence of clinical benefits. However, the company is now focused on trials aiming to achieve a functional cure of HIV infection and does not appear interested in pursuing further trials for INRs (those in the phase I study have also been denied additional rounds of infusions of gene-modified CD4 T cells, due to a claim of limited resources on the part of the company).
A group of Chinese scientists has recently published evidence that mesenchymal stem cells, which are obtained from donated umbilical cords, can significantly improve immune reconstitution and reduce immune activation in at least a subset of INRs; they are now conducting expanded studies.
Currently, the lead candidate for clinical evaluation is the cytokine IL-7, which has produced sustained increases in T-cell counts in several trials (including gut CD4 T-cell numbers), with a recent analyses also suggesting these increases are associated with declines in inflammatory biomarkers and CD4 T-cell activation. IL-7’s mechanism of action differs significantly from IL-2’s, and it lacks the latter’s notorious flu-like side effects and the bias toward inducing regulatory CD4 T cells. The manufacturer, a small Parisian Biotech company named Cytheris, is now planning a phase III trial to assess clinical outcomes among INRs.
A search of the clinicaltrials.gov database indicates that there are five studies of interventions for INRs, only one of which is in the United States (a trial of two nutritional supplements, zinc and S-adenosylmethionine [SAM-e], that is due to open for enrollment in Atlanta and Seattle). None is evaluating clinical endpoints.
The shift to recommending earlier ART initiation should reduce the incidence of INRs, but late diagnosis is an ongoing problem, and thus the unmet need of this population is unlikely to evanesce anytime soon. Advocacy efforts should remain cognizant of early ART’s potential in this regard, while also seeking to ensure that the health benefits of candidate therapies are assessed and that additional novel approaches are identified and advanced into clinical trials.•
