Rifapentine’s manufacturer helps to advance TB research while stalling access
By Erica Lessem
Sanofi-Aventis, manufacturer of the tuberculosis (TB) drug rifapentine (Priftin), can be credited for aiding research efforts to shorten and simplify treatment dosing for TB. However, the company’s pricing of the drug has hampered access to such regimens, even in resource-rich nations like the United States.
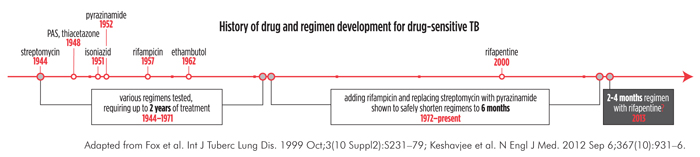
While first-line therapy for drug-sensitive TB is effective, its six-month duration and daily pill burden discourages treatment adherence and taxes health care systems. Similarly, treating latent TB infection generally requires nine months of daily treatment, meaning that many discontinue, or do not initiate. Though TB treatment has improved over time (see figure), shorter, simpler regimens for preventing and curing TB are crucial.
Rifapentine (Priftin) is an approved drug for active TB. In the same class as rifampicin—one of the backbones of first-line TB therapy—rifapentine has a longer half-life, and may be preferable for intermittent or treatment-shortening regimens. Sanofi is collaborating with the U.S. Centers for Disease Control and Prevention’s Tuberculosis Trials Consortium (TBTC), along with the International Consortium for Trials of Chemotherapeutic Agents in Tuberculosis (INTERTB), to explore simplified latent and active TB treatment regimens.
Specifically, a TBTC study showed that using rifapentine with isoniazid could shorten latent TB treatment to just 12 once-weekly doses. Another TBTC study, and a recent INTERTB study, showed that for active TB, replacing rifampicin with rifapentine in the last four months of treatment may permit once-weekly, rather than daily, dosing. The TBTC is also developing a phase III trial to determine the potential of rifapentine to shorten active TB treatment.
Implementation of these evidence-based practices has, unfortunately, been minimal—primarily due to the drug’s prohibitive pricing, compounded by major TB budget cuts. In late 2012, Sanofi lowered the U.S. federally discounted price of rifapentine from $73 to $51 per box of 32 tablets. In contrast, isoniazid costs just $0.05 per tablet. Given the potentially large market for rifapentine if the price were lowered, reductions could be cost-neutral or even lucrative for Sanofi. However, in January 2013, Sanofi rejected a request from U.S. and international TB program managers, researchers, and advocates to further lower rifapentine’s price.
The 10,000 people in the U.S. alone who get TB annually, along with hundreds of thousands of infected contacts, urgently need better treatment options—as do providers, TB programs, and taxpayers. Sanofi’s contributions to advance TB research are not meaningful if rifapentine is ultimately inaccessible. Removing cost barriers is critical to bridge the gap between TB research and practice.•
