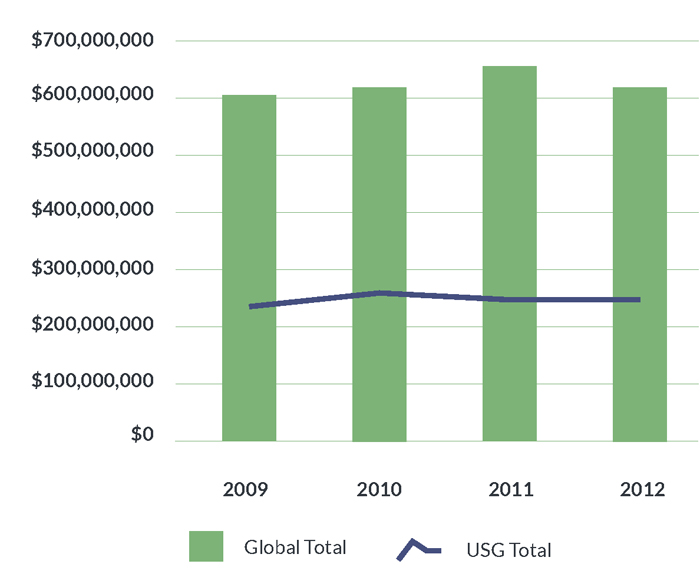
Flatlined: U.S. Government Investments in Tuberculosis Research and Development, 2009–2012
March 2014 By Mike Frick Introduction The goal of eliminating tuberculosis (TB) as a public health threat in the United States is under threat. In 1989, the U.S. government committed to ending the TB epidemic through the formation of a…