August 31, 2016
The goal of hepatitis C virus (HCV) treatment is a cure (when there is no HCV in a person’s bloodstream 12 weeks after treatment is finished).
What is Epclusa?
Epclusa is a fixed-dose combination of two HCV-fighting drugs (sofosbuvir and velpatasvir) in one pill. In the United States, Epclusa is approved for people with all hepatitis C genotypes (1–6) who are 18 years of age and older.
How is Epclusa used?
Epclusa is taken once daily, with or without food, for 12 weeks. People who have advanced (called decompensated) cirrhosis will need to add another drug, called ribavirin, twice daily. The effectiveness of the treatment depends on whether a person has cirrhosis, their virus genotype, and previous HCV treatment history.
U.S. Food and Drug Administration–Recommended Treatment Length and Cure Rates in Clinical Trials*

*Cure rates in clinical trials are higher than in the general population because the people in trials are usually healthier and receive extra monitoring and support.
The most important thing a person can do to be cured is never miss a dose of HCV treatment—this is called adherence. Adherence lowers the risk for drug resistance.
What is drug resistance?
Each day, HCV makes billions of copies of itself. Some copies are not the same as the original virus. They may have changes (called mutations) that can stop hepatitis C drugs from working. If people miss doses of their treatment, HCV gets a chance to reproduce—and some of the copies may be resistant to HCV treatment.
Some people have drug resistance even though they have never been on hepatitis C treatment—but many can be cured anyway.
Most people who are not cured have resistance to one or more of the HCV drugs they’ve taken. Resistance to some hepatitis C drugs can disappear within months, but it can also last for years and may limit re-treatment options.
Epclusa and age, gender, and race/ethnicity:
In clinical trials, cure rates did not differ by age (over 65 vs. under 65) or by gender. There is not much information about how well Epclusa works by race or ethnicity because most of the people in the trials were white. With HCV genotypes 1, 2, 4, 5, and 6 alone (no cirrhosis), black patients (98% or 51/52) were as likely as nonblack patients (99% or 564/569) to be cured with 12 weeks of Epclusa (results from the ASTRAL-1 clinical trial). Similar cure rates were also seen in black patients (3/3 or 100%) and nonblack patients (95% or 261/274) with the harder to treat genotype 3 (results from the ASTRAL-3 clinical trial).
Side effects from Epclusa:
Talk with your health care provider about possible side effects and how they can be managed. In clinical trials of Epclusa, the most common side effects were headache and fatigue, usually mild. In patients with decompensated cirrhosis, who need to take ribavirin along with Epclusa, the most common side effects were mild to moderate fatigue, anemia, nausea, headache, insomnia, and diarrhea.
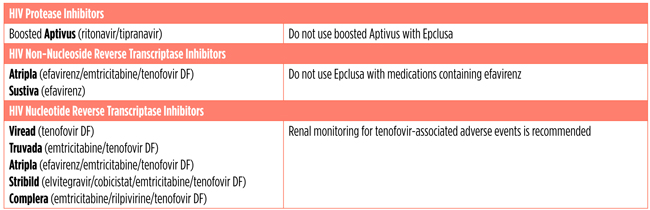
Does Epclusa work with HIV drugs?
Epclusa cannot be taken with certain HIV drugs. See Epclusa and HIV Treatments (Antiretrovirals) below for more information.
Epclusa and other medications:
Epclusa should not be used with certain drugs. Combining medications can increase or decrease drug levels (called drug-drug interactions). An increase can make side effects worse. A decrease can prevent a drug from working, putting people at risk for resistance or not being cured.
Epclusa should not be used in people taking the heart rhythm medication amiodarone because sofosbuvir, a key ingredient, can cause life-threatening heart problems. Do not take St. John’s Wort herbal supplements with Epclusa, and tell your doctor if you are taking medications for cancer, seizures, bacterial infections, heartburn/acid reflux, or statins.
Talk with your health care provider before starting or stopping any medications, supplements, or herbal remedies.
More information is available in Epclusa’s prescribing information (http://www.gilead.com/~/media/files/ pdfs/medicines/liver-disease/epclusa/epclusa_pi.pdf).
Epclusa and HIV Treatments (Antiretrovirals)
Storing Epclusa:
Keep Epclusa at room temperature (below 86°F).
Epclusa in people with kidney disease:
Epclusa can be used by people with mild or moderate kidney disease. No studies have been conducted in people with severe kidney disease (eGFR <30 mL/min/1.73 m2) or people on dialysis. Patients with severe kidney disease, or on dialysis, who also have cirrhosis should ask their doctor whether Epclusa and ribavirin are right for them.
Epclusa in people with cirrhosis:
HCV treatment guidelines recommend that people with serious liver damage (Child-Pugh Class B or C) be treated by a specialist. People with Child-Pugh Class B or C can be treated with Epclusa and ribavirin.
Epclusa during pregnancy, nursing, and in children:
It is not known whether Epclusa causes harm to unborn babies or passes into breast milk. If you are pregnant or breast feeding, or planning for either, talk with your health care provider about the risks and benefits of HCV treatment. Epclusa has not been tested in children younger than 18 years old.
Ribavirin causes birth defects and miscarriage. It should not be used by pregnant women or by male partners of pregnant women. The drug stays in a person’s body for months. Women and their male partners should avoid pregnancy for six months after they have stopped taking ribavirin. Using two forms of birth control to prevent pregnancy while taking ribavirin—and for six months afterward—is recommended. Nursing during treatment is not recommended. For more information, visit the ribavirin pregnancy registry at: http://www.ribavirinpregnancyregistry.com/.
Access to Epclusa
Access may be restricted by public and private payers. The criteria differ depending on the type of coverage and the state in which it is issued. Support Path is Gilead’s patient assistance program for Epclusa. People with private insurance may be eligible for assistance with copayments. Uninsured people may be eligible for medication at no charge. Information about Support Path is available online at: http://www.mysupportpath.com/. Information about Support Path is also available by phone at 1-855-7-MYPATH or 1-855-769-7284.
This fact sheet is current as of August 2016. Always check for updated information.

