Growing Chasm Between Bench and Bedside
Hepatitis C virus (HCV) and HIV/HCV coinfection were prominently featured at this year’s Retrovirus conference.
Coverage ranged from scientific progress in the laboratory to treatment access in the clinic. During the opening plenary, Takaji Wakita detailed the development of an important new tool for hepatitis C research: a cell culture system in which hepatitis C virus can replicate. The ability to “grow” hepatitis C virus represents a significant breakthrough with the potential to accelerate drug and vaccine development. Previously, the study of HCV replication was limited to synthetic replicon systems incapable of producing infectious particles. The only available animal model for hepatitis C research is the endangered, prohibitively expensive chimpanzee. The novel cell culture system enables the study of each step involved in viral replication. Researchers are currently using the system during preclinical drug development to identify new antiviral targets and assess activity of anti-HCV drugs.
The closing CROI Symposium, Advances in the Understanding of HCV Biology and Treatment, included a detail of the HCV protease and polymerase inhibitor pipeline. Ann Kwong from Vertex Pharmaceuticals reviewed clinical development of protease inhibitors, describing the active site of the HCV protease as a lousy target: “landing an inhibitor on the top of that enzyme is like landing on a piece of pizza — it’s just greasy and [the inhibitor] flies right off.” Previously, Boehringer Ingelheim had established proof-of-concept with BILN-2061, but development was halted in 2004 due to the drug’s cardiac toxicity. Two candidates have recently completed early Phase I studies: Vertex’s 950 and Schering’s 503034. Data appears below in Table 1.
Table 1. Antiviral Activity of Two HCV Protease Inhibitors (With or Without Peg-IFN) vs. PEG-IFN Monotherapy
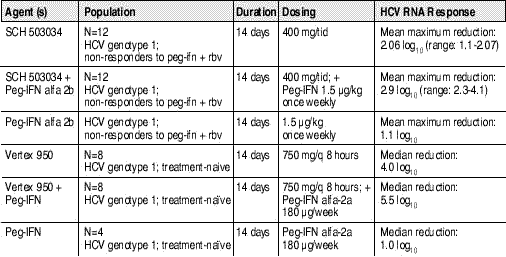
Sources: Abstracts 94 and 201, 56th Annual Meeting of the American Association for the Study of Liver Diseases; November 11-15th, 2005; San Francisco, CA; and Vertex Pharmaceuticals, press release. January 9th, 2006.
Promising results from these early trials have inspired considerable excitement and speculation. As indicated in Table 1, both PIs were active against HCV genotype 1, and in treatment na?ve persons (Vertex) and nonresponders (Schering). Genotype 1 requires 48 weeks of treatment and is less responsive to current therapy than genotypes 2, 3, and 4.
The spoiler is resistance. A single mutation at position A/156/T creates cross-resistance to HCV protease inhibitors. Additional mutations conferring low and high-level resistance to Vertex 950 have been identified. Vertex’s early research suggests that the replicative capacity of resistant virus may be poorer than that of wild-type virus. The crucial question is whether an HCV protease inhibitor-based regimen can eliminate the virus before resistance develops.
Phase I data have been used to estimate the impact of HCV protease inhibitors on hepatitis C treatment duration. These models ambitiously predict a significant treatment abbreviation from 48 to 12 weeks, but more data are needed. As Kwong rightly noted, “we’ll just have to see how it turns out.”
Daria Hazuda from Merck closed the symposium with an update on preclinical development of HCV polymerase inhibitors. HCV’s polymerase enzyme is a mother lode for anti-viral drug development; five classes of polymerase inhibitors have already been identified. Preclinical data indicate that these drugs may be combined with one another, and some may offer additive or synergistic effects. The drawback is resistance. In preclinical testing, a single amino acid change caused high-level resistance, although cross-resistance may be less likely with polymerase inhibitors than protease inhibitors.
These exciting developments bode well for the future of HCV therapy but contrast sharply with today’s clinical reality for HIV/HCV coinfected patients. Several posters at CROI documented high rates of HCV treatment ineligibility, low response to HCV therapy, and significant liver-related morbidity and mortality among coinfected persons. In particular, two HIV clinics based in Baltimore and Seattle reported that less than one third of their coinfected patients had been evaluated for HCV treatment; of the third who underwent evaluation, less than 20% began treatment. Not surprisingly, at the end of the day, the number of patients who achieved SVR during treatment represented an abysmal .7 and 1.6 percent of the entire Baltimore and Seattle-based cohorts, respectively.
Table 2. Hepatitis C Evaluation, Treatment and Response Rates at Two HIV Clinics
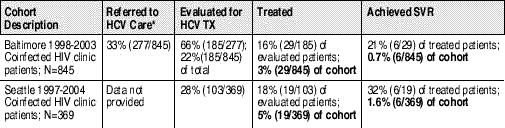
Sources: Mehta S, Lucas G, Torbenson M, et al. Barriers to referral for hepatitis C care among HIV/HCV coinfected patients in an urban HIV clinic. 13th Conference on Retrovurises and Opportunistic Infections; February 5-8th; Denver Colorado. 2006. Abstract 884;
Scott J, Wald A, Kitahata M, Drolette A, Corey L, Wang, C. HCV is evaluated and treated infrequently in an HIV/HCV coinfected population. 13th Conference on Retrovurises and Opportunistic Infections; February 5-8th; Denver Colorado. 2006. Abstract 882.
Addressing Barriers
Current barriers to HCV treatment are substantial and must be surmounted prior to the advent of new therapies. Despite accumulating evidence that HCV can be successfully treated in multiply-diagnosed, mono- and coinfected persons, some clinicians remain reluctant to treat people with histories of drug use and/or psychiatric co-morbidities. Coinfected individuals, who often reside in at least one of these two categories, have additional treatment challenges, including worse experience of side effects and toxicities, and decreased likelihood of viral clearance.
More effective, less toxic therapies will undoubtedly increase treatment uptake among coinfected people. Still, new drugs alone won’t dispel existing barriers, particularly since the clinically demanding, and difficult-to-tolerate, pegylated interferon is likely to remain the therapeutic backbone of HCV treatment for the next few years. Successful HCV treatment programs offer integrated psychiatric care, drug treatment, and include strong peer education and support components. Such programs are few and far between. The infrastructure necessary for delivering HCV treatment to coinfected people must be developed now, in anticipation of improvements in therapy.
