 By Coco Jervis
By Coco Jervis
Big changes are planned for the $300 million AIDS clinical trials networks funded by the U.S. National Institutes of Health (NIH). Founded in 1987 at the height of the AIDS crisis, the networks are credited with numerous groundbreaking clinical advances in HIV and opportunistic infection prophylaxis and treatment that has prevented countless infections and saved millions of lives. From opportunistic infection prophylaxis and combination antiretroviral (ARV) therapy, to prevention of mother-to-child transmission, to preexposure prophylaxis and antiretroviral microbicides, much of our current knowledge on how to manage and prevent HIV was tested and proven effective in trials conducted by NIH-sponsored networks in domestic and international research institutions with teams of investigators and—most important—the cooperation of tens of thousands of study volunteers. Given the centrality of these networks to AIDS research and their long history of community participation, the restructuring process is being closely watched by both researchers and AIDS activists as it will affect the research agendas and priorities for the next decade.
Accounting for approximately 10 percent of the $3 billion NIH AIDS annual budget, the networks currently have the largest portfolio of HIV/AIDS clinical research in the world. With a seven-year funding cycle set to expire at the end of 2012, the networks will be restructured to take on new and complex research challenges emerging from the evolving HIV epidemic, maintain ongoing research into vaccines and biomedical prevention, and renew emphasis on the search for a cure all the while operating under the threat of drastic U.S. congressional funding cuts in the coming years.
Emerging Research Priorities
While various treatment strategy trials such as treatment intensification and treatment interruption have not yielded favorable outcomes, the replacement of older and more toxic ARVs with newer and more tolerable drug combinations has lead to lower rates of treatment side effects and more durable, potent antiviral activity. The drug development industry has the search for novel antiretroviral compounds and classes well in hand, enabling publicly funded HIV clinical research to move away from run-of-the-mill antiretroviral drug development to focus on curing HIV, ameliorating AIDS-related aging, and focusing on pressing conditions that are killing people with HIV worldwide. These include tuberculosis (TB) and hepatitis C coinfection (HCV) and a host of comorbidities, complications and non-AIDS cancers. Recent research has established the need for investigation into the role chronic immune activation and accelerated immunosenescence play in earlier onset of aging-related conditions in people with HIV, arguing for a new multidisciplinary focus on AIDS and aging in the newly configured networks.
In order to stem HIV morbidity and mortality associated with these conditions, the networks will need to reevaluate their research priorities and bring in new leadership with expertise in these critical areas. Over the past two decades the networks’ leadership has frequently been criticized for its resistance to collaborate with outside experts, making multidisciplinary research difficult. Additionally, the mentoring and training of young investigators is desperately needed to ensure robust HIV research into the future. These are all compelling reasons for the new network structures to be more flexible to encourage cross disciplinary collaborations. While the research agenda is still a work in progress, the leadership of the National Institute of Allergy and Infectious Diseases (NIAID)—the lead NIH institute on AIDS—and its Division of AIDS (DAIDS) have highlighted key areas that they would like to focus on. The therapeutic research priorities will be on TB and HCV coinfection, and other comorbidities including those associated with HIV and aging, novel drug approaches such as weekly dosing, and working toward a cure or a functional cure, meaning viral suppression without the use of ARVs. TB and HCV will be studied both in people with and without HIV coinfection. For vaccine research, priority areas will involve phase I, II, and III vaccine strategies as well as therapeutic vaccines. In prevention research the focus will be on microbicides, preexposure prophylaxis, emerging products, and test and-treat methods. The networks will be organized to ensure infants, children, adolescents, and pregnant women are also included in all major research activity. For pediatric research, priority areas will include pharmacology and drug formulation issues as well as prevention.
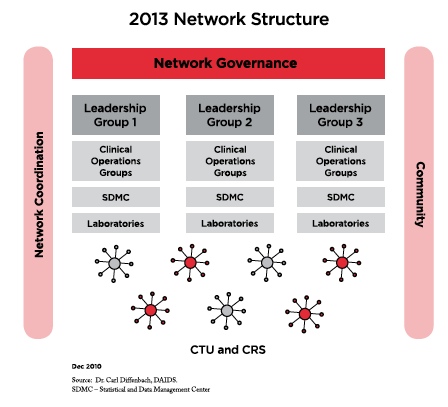
During the NIAID town hall meeting in October 2010, NIAID director Dr. Anthony S. Fauci and DAIDS head Dr. Carl Dieffenbach highlighted these coming research priorities and stated that they are seeking more transparent and collaborative mechanisms in network infrastructure and governance and are looking for innovative avenues through which to incorporate new expertise and expand community involvement. They want to create an infrastructure with multidisease research capacity so that each clinical trials unit (CTU) can be reconfigured as needed. In terms of overall network governance there will be an overarching cross cutting strategic working group that will be open to community participation to shape the vision of the network, review network strategic plans, and monitor clinical trials and transagency collaboration.
An operations working group will be responsible for implementation issues and resource utilization. Currently there are over 73 CTUs worldwide. This number is expected to be reduced by two-thirds with each new CTU overseeing four to eight clinical research sites. All CTUs will perform HIV/AIDS research, and some will be able to perform non-HIV research (e.g., research into HCV, TB, and aging). The future CTU reconfiguration may consist of two different types of sites. The first would include stable and protocol-specific clinical research sites that would have surge capacity; the second would be flexible sites with streamlined procedures for adding or eliminating clinical research sites. The reconfigured CTUs would have increased authority and accountability to perform capacity management, resource sharing, and utilization and cost-containment measures. Questions remain as to how the new structure will advance the capacity to perform HIV, HCV, and TB treatment trials internationally, particularly in resource-limited settings as well as in venues such as methadone clinics and correctional facilities.
Over the past year, the Treatment Action Group put forth recommendations to NIAID and the HIV community on network infrastructure and priority agenda-setting for HIV therapeutics, viral hepatitis, and TB. The comment period for the community is set to expire in February 2011. To read TAG’s recommendations click here. To join in the overall network restructuring discussion with your ideas or questions visit http:// blog.aids.gov/2010/06/restructuring-niaidshivaids-clinical-trials-networks.html.
