By David Branigan, Joelle Dountio Ofimboudem, and Natalie Shure
TAG was founded in 1992 with the aim of accelerating the availability of effective HIV treatments through science-based advocacy at every step of the research, development, and implementation process. These strategies helped deliver antiretroviral treatment regimens in 1996 but have required sustained action from activists, civil society, and governments to get them to the tens of millions of people around the world whose lives have been saved by these revolutionary drugs. Nonetheless, there are still hundreds of thousands of preventable AIDS deaths each year, many of which are caused by TB and HCV — two prominent HIV coinfections that TAG added to its mission in 2000 and 2012, respectively. Like HIV, both the TB and HCV pandemics disproportionately affect marginalized communities and have been worsened by political neglect, inadequate funding, and corporate greed. In a roundtable discussion moderated by Communications Coordinator Natalie Shure, HCV Acting Project Director Joelle Dountio Ofimboudem and TB Project Officer David Branigan discuss key opportunities for expanding access to care and cures moving forward:
NS: HCV and TB are both curable, but there are access problems at every level of care. What are the current challenges and future opportunities for scaling up access to key innovations that could accelerate the end of the pandemics?
JDO: For HCV we do have curative originator and generic treatments, but in a lot of countries these treatments are not accessible. HCV is not prioritized by most national health programs and millions of people with HCV remain undiagnosed. As a result, demand for treatment remains very low, rendering the market unattractive to generic developers. Moreover, the HCV diagnosis pathway in many countries remains very complex, requiring several steps, and resulting in people being lost to follow-up. One key opportunity is to simplify diagnostics to make it easier to test and treat HCV. Also, children aged three years upwards diagnosed with HCV should be treated per the World Health Organization (WHO) updated recommendations.
DB: As with HCV, much more must be done to find people with active TB. Countries have been very slow to scale up TB technologies — we see this in both treatment and diagnostics. Smear microscopy — a nineteenth century technology — persists in many places to diagnose TB, even though the WHO has recommended rapid molecular testing as the initial TB diagnostic test since 2013. To make matters worse, Cepheid held a decade-long monopoly on rapid molecular diagnostics for TB. Due to COVID-19 related lockdowns and health system disruptions, the TB diagnostic gap widened from 2.9 million people in 2019 to 4.2 million people in 2020 (out of the 10 million people estimated to develop active TB each year). Still, we’re hopeful that new tools developed for COVID-19 will have applications for TB moving forward.
NS: So what are some of the main ways of addressing these barriers? And what role will TAG play within that broader strategy?
DB: The fact that Cepheid continues to be the dominant supplier of rapid molecular diagnostics for TB and other infectious diseases has resulted in limited leverage in negotiations with the company and a lack of sufficient consequences for Cepheid’s nontransparent high pricing and inadequate service and maintenance (often leading to unacceptably long downtime between repairs). High prices of tests, equipment, and warranties have in turn contributed to slow scale-up by countries. To address these challenges, TAG joined with allied communities and civil society organizations to form the Time for $5 Coalition, which aims to push Cepheid to lower its test prices and improve service and maintenance across diseases. The Coalition also calls for donors and other global health actors to break down disease silos and pool procurement volumes across diseases to maximize bargaining power with Cepheid and other suppliers while investing in competing technologies (in addition to Molbio’s Truenat) to break the Cepheid monopoly and bring TB testing closer to the point of care.
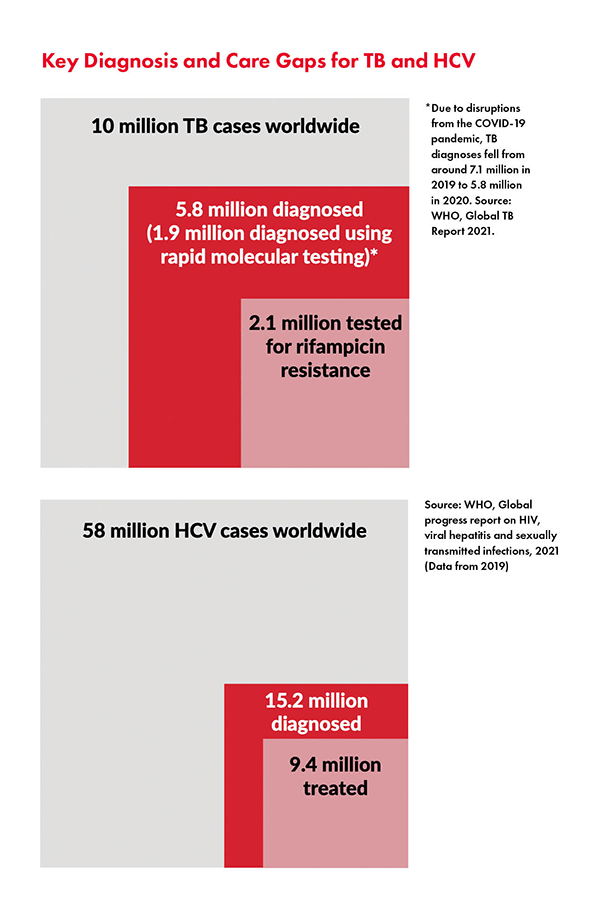 JDO: In terms of barriers for hepatitis C, governments need to start by ensuring that HCV national elimination plans include people at high risk; namely, people who use drugs, men who have sex with men, people in incarceration, immigrants, etc. Civil society needs to continue to push for decentralization of care to ensure delivery of HCV testing and treatment at community- based facilities; integration of HCV testing and treatment with existing care services at peripheral health facilities; and task sharing to ensure delivery of HCV testing, care, and treatment by trained non-specialist doctors and nurses to scale up HCV diagnosis and expand treatment access. To this end, through the Hep C PACT, TAG together with other partners is working with stakeholders in select countries to help foster an enabling environment for improved availability of HCV diagnostics and treatment in high burden low- and middle-income countries. We know that around 58 million people around the world are living with HCV, and close to 80% of these people do not know they are infected, so we must scale up diagnostics to find and treat the “missing millions” just as in TB. But without dedicated global funding for HCV elimination, direct acting antiviral (DAA) manufacturers and health ministries are less likely to act. TAG provides technical support to civil society to build community pressure as a force for change — for example, through platforms like mapCrowd and hepCoalition, codeveloped with other partners — that enable advocates to crowdsource and share key data and resources to inform their advocacy work.
JDO: In terms of barriers for hepatitis C, governments need to start by ensuring that HCV national elimination plans include people at high risk; namely, people who use drugs, men who have sex with men, people in incarceration, immigrants, etc. Civil society needs to continue to push for decentralization of care to ensure delivery of HCV testing and treatment at community- based facilities; integration of HCV testing and treatment with existing care services at peripheral health facilities; and task sharing to ensure delivery of HCV testing, care, and treatment by trained non-specialist doctors and nurses to scale up HCV diagnosis and expand treatment access. To this end, through the Hep C PACT, TAG together with other partners is working with stakeholders in select countries to help foster an enabling environment for improved availability of HCV diagnostics and treatment in high burden low- and middle-income countries. We know that around 58 million people around the world are living with HCV, and close to 80% of these people do not know they are infected, so we must scale up diagnostics to find and treat the “missing millions” just as in TB. But without dedicated global funding for HCV elimination, direct acting antiviral (DAA) manufacturers and health ministries are less likely to act. TAG provides technical support to civil society to build community pressure as a force for change — for example, through platforms like mapCrowd and hepCoalition, codeveloped with other partners — that enable advocates to crowdsource and share key data and resources to inform their advocacy work.
NS: As the COVID-19 pandemic wore on, the international push to suspend intellectual property rights to achieve global vaccine equity has not had the success that advocates hoped. How should that experience inform treatment activists’ work moving forward? How should these crises of access change the approach of civil society?
DB: For diagnostics, intellectual property isn’t currently the main barrier to access. Rather, it’s the need for increased investment in research and development and local manufacturing capacity to introduce more competition and further undermine Cepheid’s monopoly, which is also key for global vaccine equity. During the pandemic, Cepheid developed and marketed a COVID-19 test, but prioritized orders from high-income countries and undersupplied low- and middle-income countries, essentially selling to the highest bidder. This is in spite of the fact that Cepheid received over $250 million in public investment to develop GeneXpert technology, including the COVID-19 test. A similar pattern is seen across health technologies, including COVID-19 vaccines — the public invests in the development of new technologies but has limited ability to secure affordable pricing and equitable access. COVID-19 vaccine hoarding by high income countries and Cepheid’s refusal to implement lower pricing for diagnostic assays or prioritize orders for COVID-19 tests from low- and middle-income countries point to a common problem as well as a key opportunity moving foward: activists should push for the inclusion of access conditions on public funding for new technologies — conditions such as requiring transparency for evidence-based equitable pricing, volume- based price reductions, and, where appropriate, the obligation to fulfill orders from low- and middle-income countries, which was a huge problem during COVID-19. By including these conditions on funding, our hope is that this will create a healthier market that is able to meet global public health needs. To ensure that community voices most affected by infectious diseases have a seat at the table, global health donors should invest in community engagement in research and financially support civil society organizations in high-burden countries to engage communities in treatment and diagnostics literacy and advance advocacy to generate demand for new tools.
JDO: Global inequities in COVID-19 diagnostic tools, vaccines, and treatment access simply spurred the decades- long broader access to medicines and health technologies movement more generally and made it clear that the World Trade Organization is not the right forum to address these challenges. Ultimately, there’s a great need to rethink the global trade regime, and governments need to understand that. Apart from these international efforts that were basically initiated and led by civil society organizations and very few countries, governments individually also need to play their part in making use of available flexibilities included in the Trade Related Aspects of Intellectual Property Rights (TRIPS) Agreement, national intellectual property laws, and trade secret laws. So, there is a great need for civil society organizations to engage with, and push for reform with policymakers not just at the global level, but also at the local and country levels.
