By David Branigan
Ensuring access to tuberculosis (TB) preventive treatment for people most at risk of developing active TB is one of the key interventions required to end TB.1 Central to these efforts is the push to scale up short course, rifapentine-based TB preventive treatment (TPT) in countries with high burdens of TB — similar to pre-exposure prophylaxis (PrEP) for HIV. Regimens include 3HP (3 months of rifapentine and isoniazid taken weekly) and 1HP (1 month of rifapentine and isoniazid taken daily). Yet, a number of barriers have hindered uptake of rifapentine-based TPT, including limited global supply and high prices of rifapentine, substantial pill burdens of 3HP and 1HP, and concerns regarding poor adherence given TPT dosing and treatment duration requirements.2
Now, imagine a long-acting TPT regimen that can be administered in a single injection. A long-acting TPT regimen could offer several advantages over existing TPT regimens administered orally on a daily or weekly basis. These advantages include a potentially more convenient and discrete mode of drug administration than pill-taking, and improved TPT adherence and thereby effectiveness. The Unitaid-funded LONGEVITY project, led by the University of Liverpool, is in the early stages of developing long-acting forms of rifapentine and isoniazid for TB prevention. If successful, an injection into muscle or subcutaneous tissue would deliver a drug depot that would gradually release TPT into the body at a rate that provides a therapeutic concentration for weeks or even months.
Long-acting TPT could help improve TPT adherence, advance TPT scale-up among people most at risk of developing active TB, and move the world closer toward ending TB. Yet, questions remain regarding whether communities will accept long-acting injectable (LAI) TPT.
LAI TPT in the Time of All-Oral Drug-Resistant TB Treatment
The TB community only recently succeeded in advocating for the removal and replacement of toxic injectable agents from drug-resistant TB (DR-TB) regimens. Daily injections of these toxic drugs caused severe, debilitating side effects, including hearing loss.
“[T]he doctor called and said I was eligible for [DR-TB] treatment involving daily injections for two years… Although I was told about side effects, they were never monitored. After three months, I realized I was losing my hearing. I alerted the medical staff. My treatment was switched, but the deterioration continued. Today I have lost about 80% of my hearing and 60% of my sight.”3
The above testimonial of a DR-TB survivor is representative of the experiences of those who have received DR-TB treatment regimens containing injectables such as amikacin, kanamycin, or capreomycin.4 After a longstanding campaign led by TB survivors and affected communities to “Cap the Jabs,” the World Health Organization (WHO) issued updated guidance urging all countries to fully make the switch to all-oral DR-TB treatment regimens, replacing the injectable agents with bedaquiline instead.5 This follows an earlier move by the WHO to recommend against the addition of streptomycin — another injectable agent with severe adverse side effects — to first-line retreatment regimens. Instead, the WHO recommended drug-susceptibility testing to inform more optimized regimen selection.6 Memories of toxic injectables for TB treatment are still fresh. Meanwhile, advocacy for national policy changes to support access to all-oral DR-TB treatment regimens is ongoing.
While the medicines being explored for LAI TPT are not the same as the painful and toxic daily injections included in past (and in some instances presenti) DR-TB treatment regimens, negative associations among members of TB-affected communities may persist.7 Making this distinction clear and sensitizing TB-affected communities to the idea and potential advantages of new, injection-based treatment technologies for TB prevention will require robust community engagement and rigorous, community-responsive science.i
i According to the Stop TB Partnership and MSF Step Up for TB 2020 report, 46% of the 37 high-TB-burden countries surveyed still report using toxic injectables kanamycin and/or capreomycin in DR-TB treatment regimens, counter to WHO recommendations. See: https://www.msf.org/step-tb-report-2020.
Acceptability of LAI TPT Will Depend on Robust Community Engagement
Developing LAIs guided by patient preferences and values will lay the foundation for clinical trials and the future acceptability of long-acting TPT by TB-affected communities and activists. This will require engagement early on and throughout the development process to: (1) elucidate community perspectives on the acceptability of LAIs for TPT and how they will be evaluated through research; and (2) build treatment literacy in communities, including to dispel myths or false associations between LAI TPT and the toxic injectable agents now phased out of treatment for DR-TB. Community engagement will also be key to understanding the acceptability of injectables among certain high-risk populations, such as people who inject or used to inject drugs.
The LONGEVITY project determined that repositioning rifapentine and isoniazid (orally administered drugs already indicated for TB prevention) as long-acting medicines would be the most direct and least expensive approach to bringing long-acting TPT options to communities in need. Delivering doses of rifapentine and isoniazid high enough to ensure their release in adequate concentrations over an extended period is best achieved with injections.8 In the future, new chemical entities that are more potent may be more suitable for delivery via other forms of long-acting technologies, such as microneedle patches (see Figure 1).9
While the acceptability of LAI TPT requires special consideration given the history of toxic injectables for DR-TB treatment, there is precedent for LAI acceptability for HIV treatment. Two clinical trials of long-acting cabotegravir and rilpivirine (ATLAS and FLAIR) for HIV treatment showed that, while the majority of participants reported injection site reactions such as pain or tenderness, overall acceptability of LAIs was high.10,11 An editorial on the two trials noted that the results clearly showed that “the benefits of not taking a daily oral pill outweighed this inconvenience [of injection site reactions] for participants in the first year of treatment.”12
While some may prefer LAI TPT to daily or weekly oral dosing, others may prefer pills or a different form of long-acting technology. A recent informal IMPAACT4TB survey of TB activists helped to draw out this nuance. One respondent said that “[m]embers of the community would have different acceptance to LAI TPT. Some would prefer the injection, but then a number of them would not accept injections simply because they fear needles. In conclusion, to provide a client centered service, both options of TPT (tablets and injectable) should be the option.”13 Community engagement to further elucidate these preferences will be critical for ensuring that the long-acting TPT development pathway is responsive to community needs.
Clinical Trials Should Be Designed to Answer Questions Important to Communities
Acceptability of LAI TPT will depend not only on robust community engagement but also on how effectively clinical trials can demonstrate the safety, efficacy, and other potential advantages of delivering rifapentine and isoniazid via long-acting injections. While rifapentine and isoniazid have been proven safe and effective when delivered orally, especially in combination for the treatment of TB infection, both of these drugs carry risks of hepatoxicity, and rifapentine has been linked to hypersensitivity reactions in a small percentage of people who take it, a phenomenon that might be more common when rifapentine is dosed intermittently (as is the case for 3HP).14
Once LAIs are administered, if adverse events do occur, one cannot simply stop taking the medication like one can with daily pills. The long-acting medication has already been deposited and will continue to release the drug into the body.15 To reduce the risk of adverse events, cabotegravir and rilpivirine LAIs for HIV treatment are administered only after an oral lead-in period of taking the drugs in pill form for four weeks (see Figure 2).16 If an adverse event occurs within that period, the medication in pill form can be immediately discontinued to minimize its severity.
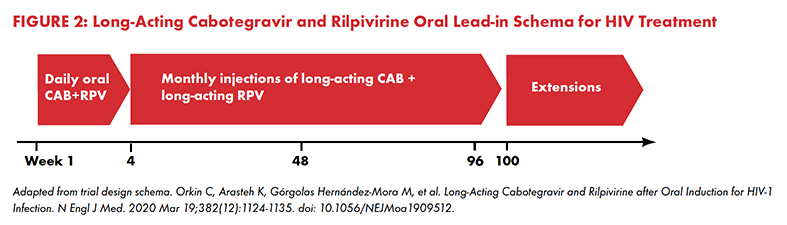
For TPT, the treatment duration may be as short as four weeks (i.e., 1HP), so is an oral lead-in necessary? If so, how long should the oral lead-in period be, and how does this affect its acceptability? What if someone who has received LAI TPT begins to show signs of hepatoxicity from the isoniazid — will there be any way to stop the release of the long-acting drug? Will the intermittent dosing of rifapentine (weekly or monthly) cause hypersensitivity reactions similar to those observed with once weekly 3HP? Rifapentine is a member of the rifamycin class of drugs, known for interactions with antiretroviral and other medications. Will LAI TPT interact with HIV antiretrovirals, hormone-based contraceptives, and methadone or buprenorphine opioid substitution therapies? These and other questions important to community acceptability must be taken into consideration and addressed in the clinical trials ahead for LAI TPT.
Preparing for the Introduction of Safe, Effective, and Acceptable Long-Acting TPT
As we engage communities and other stakeholders to prepare for future long-acting forms of TB preventive treatment, we will need to clearly explain our shift from advocating to “Cap the Jabs” to advocating for injectable TPT as a community-friendly option. To introduce safe, effective, and acceptable long-acting TPT that communities will want, the development pathway for long-acting TPT under the LONGEVITY project will need to advance in a way that is responsive both to community preferences and concerns and to science.
Endnotes
1 World Health Organization. WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment. Geneva (CH): World Health Organization; 2020 Mar. https://www.who.int/publications/i/item/9789240001503.
2 Frick M. An activist’s guide to rifapentine for the treatment of TB infection. New York: Treatment Action Group; 2020. https://www.treatmentactiongroup.org/publication/an-activists-guide-to-rifapentine-for-the-treatment-of-tb-infection/.
3 Almeida A, Adjuntsov M, Bushura W, et al. Hear Us! Accounts of people treated with injectables for drug resistant TB. Public Health Action. 2021 Sept 21;11(3):146-154(9). doi: https://doi.org/10.5588/pha.21.0031.
4 Wrohan I, Redwood L, Ho J, Velen K, Fox GJ. Ototoxicity among multidrug-resistant TB patients: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2021 Jan 1;25(1):23-30. doi: 10.5588/ijtld.20.0217.
5 World Health Organization. WHO consolidated guidelines on tuberculosis: module 4: treatment – drug-resistant tuberculosis treatment. Geneva (CH): World Health Organization; 2020 Mar. https://www.who.int/publications/i/item/9789240007048.
6 World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care: 2017 update. Geneva (CH): World Health Organization; 2017. https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf.
7 Frick M. The future of TPT is long-acting. Treatment Action Group presentation for IMPAACT4TB training. 2021 May.
8 Owen, Andrew (Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool, England). Personal communication with: David Branigan (Treatment Action Group, New York, NY). 2021 Aug 12.
9 Ibid.
10 Swindells S, Andrade-Villanueva J-F, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020 Mar 19;382:1112-1123. doi: 10.1056/NEJMoa1904398.
11 Orkin C, Arasteh K, Hernández-Mora MG, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020 Mar 19; 382:1124-1135. doi: 10.1056/NEJMoa1909512.
12 Currier J. Monthly injectable antiretroviral therapy—version 1.0 of a new treatment approach. N Engl J Med. 2020 Mar 19;382(12):1164–5. doi: 10.1056/NEJMe2002199.
13 TB activist. Treatment Action Group IMPAACT4TB community survey on LAI TPT. 2021 June 9.
14 Sterling TR, Moro RN, Borisov AS, et al. Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT tuberculosis study. Clin Infect Dis. 2015 Aug 15;61(4):527-35. doi: 10.1093%2Fcid%2Fciv323.
15 Swindells S, Siccardi M, Barrett SE, et al. Long-acting formulations for the treatment of latent tuberculosis infection: opportunities and challenges. Int J Tuberc Lung Dis. 2018 Feb 1;22(2):125–32. doi: 10.5588%2Fijtld.17.0486.
16 U.S. Food and Drug Administration. FDA approves Cabenuva and Vocabria for the treatment of HIV-1 infection. 2021 Jan 27. https://www.fda.gov/drugs/human-immunodeficiency-virus-hiv/fda-approves-cabenuva-and-vocabria-treatment-hiv-1-infection.
