Simultaneous, not sequential, evaluations of novel drug regimens needed to speed TB treatment research
By Lindsay McKenna
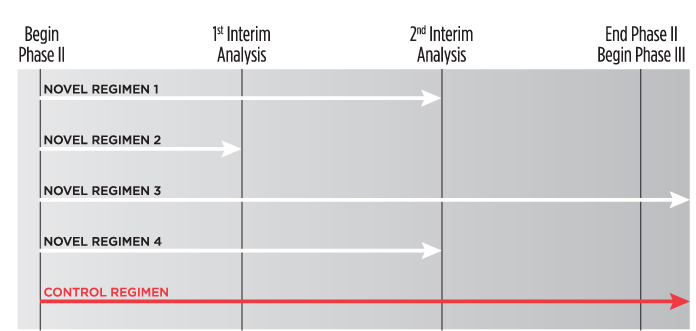
An example of a multi-arm multi-stage (MAMS) phase II trial design. At the first interim analysis, novel regimen 2 is considered to lack sufficient benefit compared with the control and is not taken forward to stage 2. At the second interim analysis, recruitment to novel regimens 1 and 4 is stopped, and only the control regimen and novel regimen 3 are continued to the end of trial and advanced into phase III studies.
New drugs, as components of novel regimens, are necessary to improve TB treatment. To expedite the development of these regimens, while simultaneously reducing the size, length, and cost of clinical trials, TB researchers, funders, and activists are working together to develop alternative study designs.
Traditional phase II studies evaluate TB regimens containing new agents sequentially—one at a time, compared with a standard control regimen— an approach that can take 20 years to yield a regimen that is desirably shorter, simpler, safer, and more effective than the existing standard of care. This process is too lengthy and expensive for the TB crisis and must be streamlined to achieve progress as fast as possible while producing rigorous data on safety and effectiveness.
Adaptive phase II clinical trials include multi-arm, multi-stage (MAMS) studies. These protocols involve simultaneous evaluation of multiple experimental regimens, using interim analyses to drop study arms deemed intolerable or ineffective based on surrogate marker data, such as time to culture conversion (see figure). The aim of such studies is to efficiently determine which novel regimens to comparatively evaluate for relapse-free cure rates in phase III trials. MAMS studies in development include the U.S. Centers for Disease Control and Prevention’s Tuberculosis Trials Consortium phase II Combination Regimens for Shortening TB Treatment (CRUSH) study, and the AIDS Clinical Trials Group’s MDR- Additive Regimens Varying Experimental Layouts (MARVEL) study.
Adaptive designs—already being used to study regimens for hepatitis C and a variety of cancers—may expedite novel TB regimen development and optimize use of limited resources. Risks include erroneous termination of an effective and safe regimen that appears ineffective or unsafe at interim analysis, and statistical difficulties in comparing multiple arms. There is also the challenge of designing novel regimens using approved and experimental drugs with overlapping toxicities, such as heart-rhythm disturbances.
Researchers should design adaptive TB drug trials, and pharmaceutical companies must make novel drugs available in combination with other compounds in development. Furthermore, regulatory authorities need to provide clear guidance on conducting adaptive clinical trials to assure quality data and participant safety—much as the U.S. Food and Drug Administration did with the release of its draft Guidance for Industry: Adaptive Design Clinical Trials for Drugs and Biologics in February 2010.
Activists can help achieve these priorities by working with research consortia to push forward adaptive designs in a way that takes into account community priorities and concerns. We also need to encourage pharmaceutical companies to provide novel drugs for adaptive trials and petition regulatory authorities to develop clear guidance on their design and implementation to ensure that such studies are scientifically sound and ethical.•
